当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nickel-Catalyzed Sodium Hypophosphite-Participated Direct Hydrophosphonylation of Alkyne toward H-Phosphinates
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-02-24 , DOI: 10.1021/acs.joc.2c02741 Dang-Wei Qian 1 , Jin Yang 1 , Gang-Wei Wang 1 , Shang-Dong Yang 1, 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-02-24 , DOI: 10.1021/acs.joc.2c02741 Dang-Wei Qian 1 , Jin Yang 1 , Gang-Wei Wang 1 , Shang-Dong Yang 1, 2
Affiliation
|
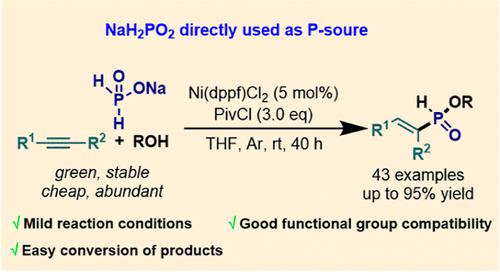
The traditional methods for the synthesis of phosphinate esters use phosphorus trichloride (PCl3) as the phosphorous source, resulting in procedures that are often highly polluting and energy intensive. The search for an alternative approach that is both mild and environmentally friendly is a challenging, yet highly rewarding task in modern chemistry. Herein, we use an inorganic phosphorous-containing species, NaH2PO2, to serve as the source of phosphorous that participates directly in the nickel-catalyzed selective alkyne hydrophosphonylation reaction. The transformation was achieved in a multicomponent fashion and at room temperature, and most importantly, the H-phosphinate product generated is an advanced intermediate which can be readily converted into diverse phosphinate derivatives, including those bearing new P–C, P–S, P–N, P–Se, and P–O bonds, thus providing a complimentary method to classic phosphinate ester synthesis techniques.
中文翻译:

镍催化次磷酸钠参与炔烃直接氢化磷酸化生成 H-次膦酸盐
传统的亚膦酸酯合成方法使用三氯化磷 (PCl 3 ) 作为磷源,导致过程污染严重且能源密集。寻找一种既温和又环保的替代方法是现代化学中一项具有挑战性但回报丰厚的任务。在此,我们使用无机含磷物质 NaH 2 PO 2作为磷源,直接参与镍催化的选择性炔烃氢膦酰化反应。这种转变是在室温下以多组分方式实现的,最重要的是,H- 生成的次膦酸盐产品是一种高级中间体,可以很容易地转化为多种次膦酸盐衍生物,包括带有新 P-C、P-S、P-N、P-Se 和 P-O 键的衍生物,从而提供一种补充方法经典的次膦酸酯合成技术。
更新日期:2023-02-24
中文翻译:

镍催化次磷酸钠参与炔烃直接氢化磷酸化生成 H-次膦酸盐
传统的亚膦酸酯合成方法使用三氯化磷 (PCl 3 ) 作为磷源,导致过程污染严重且能源密集。寻找一种既温和又环保的替代方法是现代化学中一项具有挑战性但回报丰厚的任务。在此,我们使用无机含磷物质 NaH 2 PO 2作为磷源,直接参与镍催化的选择性炔烃氢膦酰化反应。这种转变是在室温下以多组分方式实现的,最重要的是,H- 生成的次膦酸盐产品是一种高级中间体,可以很容易地转化为多种次膦酸盐衍生物,包括带有新 P-C、P-S、P-N、P-Se 和 P-O 键的衍生物,从而提供一种补充方法经典的次膦酸酯合成技术。






























 京公网安备 11010802027423号
京公网安备 11010802027423号