当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Taming Bromine Azide for Use in Organic Solvents─Radical Bromoazidations and Alcohol Oxidations
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-02-23 , DOI: 10.1021/acs.joc.2c03012 Göran Schulz 1 , Vincent George 1 , Daghan Taser 1 , Andreas Kirschning 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2023-02-23 , DOI: 10.1021/acs.joc.2c03012 Göran Schulz 1 , Vincent George 1 , Daghan Taser 1 , Andreas Kirschning 1
Affiliation
|
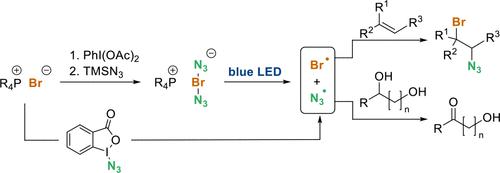
The formation of bromine azide from the bisazidobromate(I) anion or alternatively from Zhdankin’s reagent, using a phosphonium bromide salt as a common starting point, is reported. After homolytic cleavage in the presence of alkenes or alcohols either 1,2-functionalization or alternatively the selective oxidation of secondary alcohols in the presence of primary alcohols occur. The scopes and limitations of the use of BrN3 are covered.
中文翻译:

驯服溴叠氮化物用于有机溶剂─自由基溴叠氮化和醇氧化
据报道,使用溴化磷盐作为共同起点,从双叠氮基溴酸盐 (I) 阴离子或从 Zhdankin 试剂形成溴叠氮化物。在烯烃或醇存在下均裂后,1,2-官能化或仲醇在伯醇存在下发生选择性氧化。涵盖了BrN 3使用的范围和限制。
更新日期:2023-02-23
中文翻译:

驯服溴叠氮化物用于有机溶剂─自由基溴叠氮化和醇氧化
据报道,使用溴化磷盐作为共同起点,从双叠氮基溴酸盐 (I) 阴离子或从 Zhdankin 试剂形成溴叠氮化物。在烯烃或醇存在下均裂后,1,2-官能化或仲醇在伯醇存在下发生选择性氧化。涵盖了BrN 3使用的范围和限制。





























 京公网安备 11010802027423号
京公网安备 11010802027423号