当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enzymatic α-Ketothioester Decarbonylation Occurs in the Assembly Line of Barbamide for Skeleton Editing
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-02-23 , DOI: 10.1021/jacs.2c10277 Shengjie Guo 1 , Yueqian Sang 2 , Chao Zheng 3 , Xiao-Song Xue 2 , Zhijun Tang 1 , Wen Liu 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-02-23 , DOI: 10.1021/jacs.2c10277 Shengjie Guo 1 , Yueqian Sang 2 , Chao Zheng 3 , Xiao-Song Xue 2 , Zhijun Tang 1 , Wen Liu 1
Affiliation
|
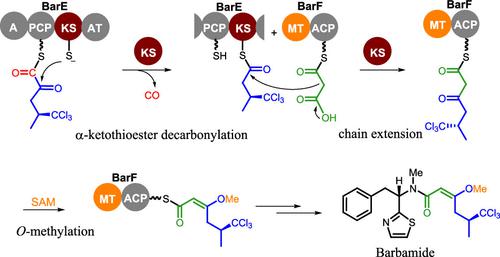
The decarbonylation reaction has been developed significantly in organic chemistry as an effective approach to various synthetic applications, but enzymatic precedents for this reaction are rare. Based on investigations into the hybrid nonribosomal peptide synthetase (NRPS)-polyketide synthase (PKS) assembly line of barbamide, we report an on-line α-ketothioester decarbonylation reaction that leads to one-carbon truncation of the elongating skeleton. This enzymatic editing reaction occurs in the first round of lipopeptide extension and modification involving the multienzymes BarE and BarF, which successively house an NRPS module to initiate the biosynthesis and a PKS module to catalyze the first round of chain extension. Starting with processing a leucine-derived α-ketoacyl starter, the ketosynthase domain in BarE displays an unusual dual activity that results in net one-carbon chain elongation. It extrudes carbon monoxide from α-keto-isocaproyl thioester and then mediates decarboxylative condenses of the resultant isovaleryl thioester with malonyl thioester to form a diketide intermediate, followed by BarF-based O-methylation to stabilize the enol form of the β-carbonyl and afford an unusual E-double bond. Biochemical characterization, chemical synthesis, computational analysis, and the experimental outcome of site-directed mutagenesis illustrate the extraordinary catalytic capability of this ketosynthase domain. This work furthers the appreciation of assembly line chemistry and opens the door to new approaches for skeleton editing/engineering of related molecules using synthetic biology approaches.
中文翻译:

用于骨架编辑的 Barbamide 装配线中发生酶促 α-酮硫酯脱羰基作用
作为各种合成应用的有效方法,脱羰反应在有机化学中得到了显着发展,但这种反应的酶促先例很少见。基于对杂合非核糖体肽合成酶 (NRPS)-聚酮化合物合酶 (PKS) barbamide 装配线的研究,我们报告了一个在线 α-酮硫酯脱羰基反应,该反应导致延伸骨架的一个碳截断。这种酶促编辑反应发生在涉及多酶 BarE 和 BarF 的第一轮脂肽延伸和修饰中,它们依次包含一个 NRPS 模块以启动生物合成和一个 PKS 模块以催化第一轮链延伸。从加工亮氨酸衍生的 α-酮酰基起始剂开始,BarE 中的酮合成酶结构域显示出不寻常的双重活性,导致净单碳链伸长。它从α-酮基异己酰硫酯中挤出一氧化碳,然后介导所得异戊酰硫酯与丙二酰硫酯脱羧缩合形成二酮化合物中间体,然后以 BarF 为基础O-甲基化以稳定 β-羰基的烯醇形式并提供不寻常的E-双键。生化表征、化学合成、计算分析和定点诱变的实验结果说明了这种酮合成酶结构域的非凡催化能力。这项工作进一步加深了对流水线化学的认识,并为使用合成生物学方法对相关分子进行骨架编辑/工程化的新方法打开了大门。
更新日期:2023-02-23
中文翻译:

用于骨架编辑的 Barbamide 装配线中发生酶促 α-酮硫酯脱羰基作用
作为各种合成应用的有效方法,脱羰反应在有机化学中得到了显着发展,但这种反应的酶促先例很少见。基于对杂合非核糖体肽合成酶 (NRPS)-聚酮化合物合酶 (PKS) barbamide 装配线的研究,我们报告了一个在线 α-酮硫酯脱羰基反应,该反应导致延伸骨架的一个碳截断。这种酶促编辑反应发生在涉及多酶 BarE 和 BarF 的第一轮脂肽延伸和修饰中,它们依次包含一个 NRPS 模块以启动生物合成和一个 PKS 模块以催化第一轮链延伸。从加工亮氨酸衍生的 α-酮酰基起始剂开始,BarE 中的酮合成酶结构域显示出不寻常的双重活性,导致净单碳链伸长。它从α-酮基异己酰硫酯中挤出一氧化碳,然后介导所得异戊酰硫酯与丙二酰硫酯脱羧缩合形成二酮化合物中间体,然后以 BarF 为基础O-甲基化以稳定 β-羰基的烯醇形式并提供不寻常的E-双键。生化表征、化学合成、计算分析和定点诱变的实验结果说明了这种酮合成酶结构域的非凡催化能力。这项工作进一步加深了对流水线化学的认识,并为使用合成生物学方法对相关分子进行骨架编辑/工程化的新方法打开了大门。






























 京公网安备 11010802027423号
京公网安备 11010802027423号