当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reductive Asymmetric Aza-Mislow-Evans Rearrangement by 1,3,2-Diazaphospholene Catalysis**
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2023-02-23 , DOI: 10.1002/anie.202301076
Guoting Zhang 1 , Nicolai Cramer 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2023-02-23 , DOI: 10.1002/anie.202301076
Guoting Zhang 1 , Nicolai Cramer 1
Affiliation
|
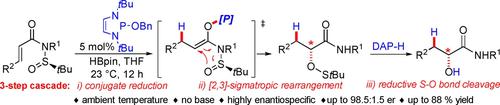
A 1,3,2-diazaphospholene-catalyzed three-step cascade transformation of chiral N-sulfinyl acrylamides comprised of a conjugate reduction, enantiospecific [2,3]-sigmatropic aza-Mislow-Evans rearrangement, and subsequent S−O bond reductive cleavage provides access to synthetically valuable enantio-enriched α-hydroxy amides. Various α-hydroxy amides are obtained in good yields and high enantioselectivities under very mild conditions.
中文翻译:

通过 1,3,2-二氮杂磷烯催化还原不对称 Aza-Mislow-Evans 重排**
1,3,2-二氮杂磷烯催化的手性N-亚磺酰丙烯酰胺的三步级联转化,包括共轭还原、对映特异性 [2,3]-sigmatropic aza-Mislow-Evans 重排和随后的 S−O 键还原裂解提供了获得具有合成价值的富含对映体的 α-羟基酰胺的途径。在非常温和的条件下,以高产率和高对映选择性获得各种 α-羟基酰胺。
更新日期:2023-02-23
中文翻译:

通过 1,3,2-二氮杂磷烯催化还原不对称 Aza-Mislow-Evans 重排**
1,3,2-二氮杂磷烯催化的手性N-亚磺酰丙烯酰胺的三步级联转化,包括共轭还原、对映特异性 [2,3]-sigmatropic aza-Mislow-Evans 重排和随后的 S−O 键还原裂解提供了获得具有合成价值的富含对映体的 α-羟基酰胺的途径。在非常温和的条件下,以高产率和高对映选择性获得各种 α-羟基酰胺。

































 京公网安备 11010802027423号
京公网安备 11010802027423号