当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Chiral Sulfonimidoyl Chloride via Desymmetrizing Enantioselective Hydrolysis
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-02-22 , DOI: 10.1021/jacs.2c13758 Gao-Feng Yang 1 , Yi Yuan 1 , Yin Tian 2 , Shi-Qi Zhang 1 , Xin Cui 1 , Bing Xia 1 , Guang-Xun Li 1 , Zhuo Tang 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-02-22 , DOI: 10.1021/jacs.2c13758 Gao-Feng Yang 1 , Yi Yuan 1 , Yin Tian 2 , Shi-Qi Zhang 1 , Xin Cui 1 , Bing Xia 1 , Guang-Xun Li 1 , Zhuo Tang 1
Affiliation
|
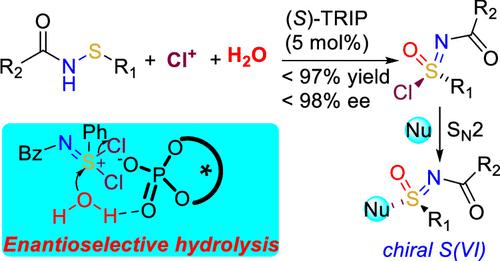
Direct construction of chiral S(VI) from prochiral S(II) is a formidable challenge due to the inevitable formation of stable chiral S(IV). Previous synthetic strategies rely on the conversion of chiral S(IV) or enantioselective desymmetrization of preformed symmetrical S(VI) substrates. Here, we report desymmetrizing enantioselective hydrolysis of in situ-generated symmetric aza-dichlorosulfonium from sulfenamides for the preparation of chiral sulfonimidoyl chlorides, which could be used as a general stable synthon for obtaining a series of chiral S(VI) derivatives.
中文翻译:

去对称对映选择性水解合成手性亚磺酰氯
由于不可避免地形成稳定的手性 S(IV),从前手性 S(II) 直接构建手性 S(VI) 是一项艰巨的挑战。以前的合成策略依赖于手性 S(IV) 的转化或预先形成的对称 S(VI) 底物的对映选择性去对称化。在这里,我们报道了从次磺酰胺原位生成的对称氮杂-二氯硫鎓的去对称对映选择性水解,用于制备手性亚磺酰亚胺酰氯,它可以用作获得一系列手性 S(VI) 衍生物的通用稳定合成子。
更新日期:2023-02-22
中文翻译:

去对称对映选择性水解合成手性亚磺酰氯
由于不可避免地形成稳定的手性 S(IV),从前手性 S(II) 直接构建手性 S(VI) 是一项艰巨的挑战。以前的合成策略依赖于手性 S(IV) 的转化或预先形成的对称 S(VI) 底物的对映选择性去对称化。在这里,我们报道了从次磺酰胺原位生成的对称氮杂-二氯硫鎓的去对称对映选择性水解,用于制备手性亚磺酰亚胺酰氯,它可以用作获得一系列手性 S(VI) 衍生物的通用稳定合成子。































 京公网安备 11010802027423号
京公网安备 11010802027423号