当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Isolated Organometallic Nickel(III) and Nickel(IV) Complexes Relevant to Carbon–Carbon Bond Formation Reactions
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2016-09-21 , DOI: 10.1021/jacs.6b06862 Jason W. Schultz 1 , Kei Fuchigami 1 , Bo Zheng 1 , Nigam P. Rath 2 , Liviu M. Mirica 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2016-09-21 , DOI: 10.1021/jacs.6b06862 Jason W. Schultz 1 , Kei Fuchigami 1 , Bo Zheng 1 , Nigam P. Rath 2 , Liviu M. Mirica 1
Affiliation
|
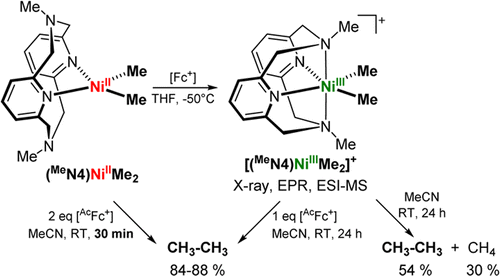
Nickel-catalyzed cross-coupling reactions are experiencing a dramatic resurgence in recent years given their ability to employ a wider range of electrophiles as well as promote stereospecific or stereoselective transformations. In contrast to the extensively studied Pd catalysts that generally employ diamagnetic intermediates, Ni systems can more easily access various oxidation states including odd-electron configurations. For example, organometallic NiIII intermediates with aryl and/or alkyl ligands are commonly proposed as the active intermediates in cross-coupling reactions. Herein, we report the first isolated NiIII-dialkyl complex and show that this species is involved in stoichiometric and catalytic C-C bond formation reactions. Interestingly, the rate of C-C bond formation from a NiIII center is enhanced in the presence of an oxidant, suggesting the involvement of transient NiIV species. Indeed, such a NiIV species was observed and characterized spectroscopically for a nickelacycle system. Overall, these studies suggest that both NiIII and NiIV species could play an important role in a range of Ni-catalyzed cross-coupling reactions, especially those involving alkyl substrates.
中文翻译:

与碳-碳键形成反应相关的分离的有机金属镍 (III) 和镍 (IV) 配合物
鉴于镍催化的交叉偶联反应能够使用更广泛的亲电子试剂以及促进立体有择或立体选择性转化,近年来它们正在经历戏剧性的复苏。与广泛研究的通常采用抗磁性中间体的 Pd 催化剂相比,Ni 系统可以更容易地获得各种氧化态,包括奇电子构型。例如,具有芳基和/或烷基配体的有机金属 NiIII 中间体通常被认为是交叉偶联反应中的活性中间体。在此,我们报告了第一个分离的 NiIII-二烷基复合物,并表明该物种参与化学计量和催化 CC 键形成反应。有趣的是,在氧化剂的存在下,NiIII 中心形成 CC 键的速率增加,表明瞬态 NiIV 物种的参与。事实上,对于镍环系统,这样的 NiIV 物种被观察到并通过光谱表征。总的来说,这些研究表明 NiIII 和 NiIV 物质都可以在一系列 Ni 催化的交叉偶联反应中发挥重要作用,尤其是那些涉及烷基底物的反应。
更新日期:2016-09-21
中文翻译:

与碳-碳键形成反应相关的分离的有机金属镍 (III) 和镍 (IV) 配合物
鉴于镍催化的交叉偶联反应能够使用更广泛的亲电子试剂以及促进立体有择或立体选择性转化,近年来它们正在经历戏剧性的复苏。与广泛研究的通常采用抗磁性中间体的 Pd 催化剂相比,Ni 系统可以更容易地获得各种氧化态,包括奇电子构型。例如,具有芳基和/或烷基配体的有机金属 NiIII 中间体通常被认为是交叉偶联反应中的活性中间体。在此,我们报告了第一个分离的 NiIII-二烷基复合物,并表明该物种参与化学计量和催化 CC 键形成反应。有趣的是,在氧化剂的存在下,NiIII 中心形成 CC 键的速率增加,表明瞬态 NiIV 物种的参与。事实上,对于镍环系统,这样的 NiIV 物种被观察到并通过光谱表征。总的来说,这些研究表明 NiIII 和 NiIV 物质都可以在一系列 Ni 催化的交叉偶联反应中发挥重要作用,尤其是那些涉及烷基底物的反应。






























 京公网安备 11010802027423号
京公网安备 11010802027423号