当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical Insight into Oxidation of Anilines to Azobenzenes Catalyzed by Hexamolybdate: Outer-Sphere Electron and Proton Transfer
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2023-02-21 , DOI: 10.1021/acs.jpcc.3c00035
Xiaofang Su 1 , Yongge Wei 2 , Nana Ma 1 , Hucheng Zhang 1 , Likai Yan 3
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2023-02-21 , DOI: 10.1021/acs.jpcc.3c00035
Xiaofang Su 1 , Yongge Wei 2 , Nana Ma 1 , Hucheng Zhang 1 , Likai Yan 3
Affiliation
|
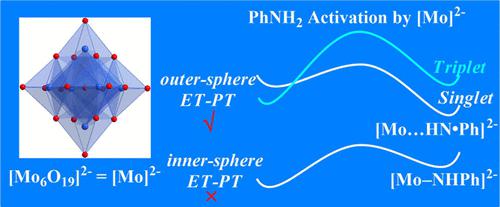
Recently, the catalytic activity of Lindqvist-type hexamolybdate [Mo6O19]2– in the oxidation of an aniline derivative (LPhNH2, L = substituent) was demonstrated by Wei and co-workers (Angew. Chem. Int. Ed. 2021, 60, 6382–6385). Herein, taking phenylamine (PhNH2) oxidation to azobenzene (PhNNPh) as a model reaction, we report the density functional theory investigation of the catalytic mechanism of [Mo6O19]2– and illustrate the critical experimental phenomena. During the catalytic reaction, once the preassociation of [Mo6O19]2– and PhNH2 takes place, electron transfer and proton transfer immediately proceed to form an N radical intermediate. The higher the highest occupied molecular orbital energy of the substrate, the easier the formation of the N radical intermediate. The N–N bond formation proceeds via the second PhNH2 nucleophilic attack on the N radical intermediate. The substituent position and the N reaction site of the substrate have a significant effect on the second PhNH2 nucleophilic attack process. In the reaction process, the six MoVI of [Mo6O19]2– are still hexacoordinated, which is defined as the outer-sphere pathway. One of the factors determining the product selectivity is the electrostatic repulsion between LPhNH2 and the N radical intermediate. The experiment reveals that the product yield is increased by the addition of Na2S2O3, while the catalytic reaction is completely deactivated with Na2CO3 or K3PO4. Based on the proposed mechanism, the experimental observation was rationalized. The S2O32– part of Na2S2O3 has a similar function as the electron-withdrawing substituent due to its low lowest unoccupied molecular orbital (LUMO) energy, which reduces the LUMO energy of the N radical intermediate and thus facilitates PhNH2 nucleophilic attack, while the CO32– part of Na2CO3 or PO43– part of K3PO4 has an undesirable effect on the electrophilicity of the N radical intermediate, resulting in the interruption of the catalytic reaction. This work would provide a detailed understanding of the catalytic reaction.
中文翻译:

六钼酸盐催化苯胺氧化成偶氮苯的理论洞察:外层电子和质子转移
最近,Wei 及其同事 ( Angew . Chem . Int . Ed. 2021, 60, 6382–6385 ). 在此,我们以苯胺 (PhNH 2 ) 氧化成偶氮苯 (PhNNPh) 作为模型反应,报告了 [Mo 6 O 19 ] 2–催化机理的密度泛函理论研究,并说明了关键的实验现象。在催化反应过程中,一旦[Mo 6 O 19 ] 2–和PhNH 2发生时,电子转移和质子转移立即进行,形成N自由基中间体。底物最高占据分子轨道能越高,越容易形成N自由基中间体。N-N 键的形成通过对 N 自由基中间体的第二次 PhNH2 亲核攻击进行。底物的取代基位置和N反应位点对PhNH2的第二次亲核攻击过程有显着影响。在反应过程中, [Mo 6 O 19 ] 2–的六个 Mo VI仍然是六配位的,这被定义为外球通路。LPhNH 2与N自由基中间体之间的静电排斥是决定产物选择性的因素之一。实验表明,通过添加Na 2 S 2 O 3提高了产物产率,而用Na 2 CO 3或K 3 PO 4使催化反应完全失活。基于所提出的机制,对实验观察进行了合理化。S 2 O 3 2– Na 2 S 2 O 3的一部分由于其最低未占分子轨道 (LUMO) 能量低,具有与吸电子取代基类似的功能,这降低了 N 自由基中间体的 LUMO 能量,从而促进 PhNH 2 的亲核攻击,而CO 3 2–部分Na 2 CO 3或 PO 4 3– K 3 PO 4的一部分对 N 自由基中间体的亲电性有不良影响,导致催化反应中断。这项工作将提供对催化反应的详细了解。
更新日期:2023-02-21
中文翻译:

六钼酸盐催化苯胺氧化成偶氮苯的理论洞察:外层电子和质子转移
最近,Wei 及其同事 ( Angew . Chem . Int . Ed. 2021, 60, 6382–6385 ). 在此,我们以苯胺 (PhNH 2 ) 氧化成偶氮苯 (PhNNPh) 作为模型反应,报告了 [Mo 6 O 19 ] 2–催化机理的密度泛函理论研究,并说明了关键的实验现象。在催化反应过程中,一旦[Mo 6 O 19 ] 2–和PhNH 2发生时,电子转移和质子转移立即进行,形成N自由基中间体。底物最高占据分子轨道能越高,越容易形成N自由基中间体。N-N 键的形成通过对 N 自由基中间体的第二次 PhNH2 亲核攻击进行。底物的取代基位置和N反应位点对PhNH2的第二次亲核攻击过程有显着影响。在反应过程中, [Mo 6 O 19 ] 2–的六个 Mo VI仍然是六配位的,这被定义为外球通路。LPhNH 2与N自由基中间体之间的静电排斥是决定产物选择性的因素之一。实验表明,通过添加Na 2 S 2 O 3提高了产物产率,而用Na 2 CO 3或K 3 PO 4使催化反应完全失活。基于所提出的机制,对实验观察进行了合理化。S 2 O 3 2– Na 2 S 2 O 3的一部分由于其最低未占分子轨道 (LUMO) 能量低,具有与吸电子取代基类似的功能,这降低了 N 自由基中间体的 LUMO 能量,从而促进 PhNH 2 的亲核攻击,而CO 3 2–部分Na 2 CO 3或 PO 4 3– K 3 PO 4的一部分对 N 自由基中间体的亲电性有不良影响,导致催化反应中断。这项工作将提供对催化反应的详细了解。

































 京公网安备 11010802027423号
京公网安备 11010802027423号