当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
4H-Pyrido[1,2-a]pyrimidin-4-one, biologically important fused heterocyclic scaffold: Synthesis and functionalization
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2023-02-19 , DOI: 10.1002/jhet.4637 Rajesh T. Bhawale 1 , Abhinay S. Chillal 1 , Umesh A. Kshirsagar 1
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2023-02-19 , DOI: 10.1002/jhet.4637 Rajesh T. Bhawale 1 , Abhinay S. Chillal 1 , Umesh A. Kshirsagar 1
Affiliation
|
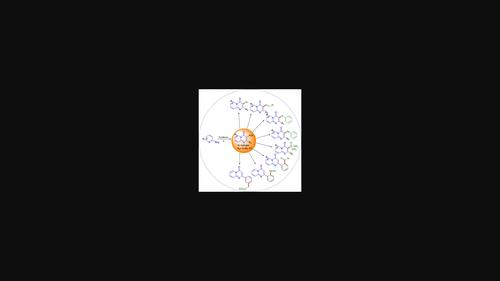
4H-pyrido[1,2-a]pyrimidin-4-one and their derivatives are highly bioactive and multipurpose heterocyclic motifs, having application in drugs, natural products, agrochemical, material science, and organic synthesis. The C-H functionalization of basic core structure of heterocycles have become indispensable tools that have continually increased the importance in the pharmaceutical industry for the synthesis of various useful heterocyclic molecules and their derivatives. In this review, we have provided a summary of various routes to synthesize 4H-pyrido[1,2-a]pyrimidin-4-one derivatives along with various derivatization methods such as via cross-coupling, C-H functionalization through arylation, alkenylation, sulfenylation, selenylation, phosphonation etc. taking into account the synthetic potentiality and significant influence of this scaffold in drug discovery.
中文翻译:

4H-吡啶并[1,2-a]嘧啶-4-一,生物学上重要的稠合杂环支架:合成和功能化
4 H-吡啶并[1,2- a ]嘧啶-4-酮及其衍生物是具有高度生物活性和多用途的杂环基序,在药物、天然产物、农用化学品、材料科学和有机合成中具有应用。杂环基本核心结构的CH官能化已成为不可或缺的工具,其在制药工业中合成各种有用的杂环分子及其衍生物的重要性不断提高。在这篇综述中,我们总结了合成 4 H -pyrido[1,2- a的各种路线]pyrimidin-4-one 衍生物以及各种衍生化方法,例如通过交叉偶联,通过芳基化、烯基化、磺基化、硒基化、磷酸化等进行 CH 官能化,考虑到该支架在药物发现中的合成潜力和重大影响。
更新日期:2023-02-19
中文翻译:

4H-吡啶并[1,2-a]嘧啶-4-一,生物学上重要的稠合杂环支架:合成和功能化
4 H-吡啶并[1,2- a ]嘧啶-4-酮及其衍生物是具有高度生物活性和多用途的杂环基序,在药物、天然产物、农用化学品、材料科学和有机合成中具有应用。杂环基本核心结构的CH官能化已成为不可或缺的工具,其在制药工业中合成各种有用的杂环分子及其衍生物的重要性不断提高。在这篇综述中,我们总结了合成 4 H -pyrido[1,2- a的各种路线]pyrimidin-4-one 衍生物以及各种衍生化方法,例如通过交叉偶联,通过芳基化、烯基化、磺基化、硒基化、磷酸化等进行 CH 官能化,考虑到该支架在药物发现中的合成潜力和重大影响。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号