当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective Photocatalytic Conversion of Methane Induced by Lewis Acid–Base Pair on the Surface of In2O3/TiO2 Heterojunction Photocatalyst
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2023-02-21 , DOI: 10.1021/acssuschemeng.2c05045 Yanmei Chen 1, 2 , Sishi Tang 1 , Li Li 1 , Xingman Liu 1 , Jun Liang 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2023-02-21 , DOI: 10.1021/acssuschemeng.2c05045 Yanmei Chen 1, 2 , Sishi Tang 1 , Li Li 1 , Xingman Liu 1 , Jun Liang 1
Affiliation
|
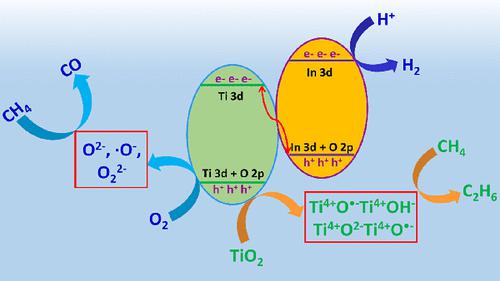
The efficient catalytic conversion of light-driven methane (CH4) is still very challenging. Here, the surface-reconstructed In2O3/TiO2 heterojunction photocatalyst is successfully prepared by a two-step operation of ion exchange and calcination, and the efficient selective photocatalytic conversion of CH4 is achieved. In the absence of O2, the formation rates of ethane (C2H6) and hydrogen (H2) are 68.9 and 78.6 μmol h–1 g–1, respectively, which corresponds to the stoichiometric ratio. However, in the presence of O2 or water (H2O) as the oxidant, CH4 is converted to CO, and the CO formation rates are 113.2 and 94.4 μmol h–1 g–1, respectively. Our results show that the In3–O2– Lewis acid–base pair on the In2O3/TiO2 surface can realize the adsorption and activation of CH4. Moreover, the photogenerated hole-induced reactive oxygen centers (Ti4+O·–Ti4+OH– and Ti4+O2–Ti4+O·–) on the surface of In2O3/TiO2 are identified as the active species for inducing the dissociation of CH4 into ·CH3 radicals, which in turn are coupled into C2H6 by ·CH3. In contrast, in the presence of O2, the surface superoxide anion (O2–), surface ·O– radicals, and O22– anions are identified as the active species for inducing the oxidation of CH4 into CO.
中文翻译:

In2O3/TiO2异质结光催化剂表面路易斯酸碱对诱导甲烷选择性光催化转化
光驱动甲烷(CH 4)的高效催化转化仍然非常具有挑战性。在此,通过离子交换和煅烧两步操作成功制备了表面重构的In 2 O 3 /TiO 2异质结光催化剂,实现了CH 4的高效选择性光催化转化。在没有 O 2的情况下,乙烷 (C 2 H 6 ) 和氢气 (H 2 )的生成速率分别为 68.9 和 78.6 μmol h –1 g –1,这与化学计量比相对应。然而,在 O 2或水(H2 O)作为氧化剂,CH 4转化为CO,CO生成率分别为113.2和94.4 μmol h –1 g –1。我们的研究结果表明, In 2 O 3 /TiO 2表面上的In 3 –O 2 –路易斯酸碱对可以实现CH 4的吸附和活化。此外,In 2表面的光生空穴诱导活性氧中心(Ti 4+ O ·– Ti 4+ OH –和 Ti 4+ O 2– Ti 4+ O ·– )O 3 /TiO 2被确定为诱导CH 4离解成·CH 3自由基的活性物质,而后者又通过·CH 3偶联成C 2 H 6。相反,在 O 2存在下,表面超氧阴离子 (O 2 – )、表面·O –自由基和 O 2 2–阴离子被确定为诱导 CH 4氧化成 CO 的活性物质。
更新日期:2023-02-21
中文翻译:

In2O3/TiO2异质结光催化剂表面路易斯酸碱对诱导甲烷选择性光催化转化
光驱动甲烷(CH 4)的高效催化转化仍然非常具有挑战性。在此,通过离子交换和煅烧两步操作成功制备了表面重构的In 2 O 3 /TiO 2异质结光催化剂,实现了CH 4的高效选择性光催化转化。在没有 O 2的情况下,乙烷 (C 2 H 6 ) 和氢气 (H 2 )的生成速率分别为 68.9 和 78.6 μmol h –1 g –1,这与化学计量比相对应。然而,在 O 2或水(H2 O)作为氧化剂,CH 4转化为CO,CO生成率分别为113.2和94.4 μmol h –1 g –1。我们的研究结果表明, In 2 O 3 /TiO 2表面上的In 3 –O 2 –路易斯酸碱对可以实现CH 4的吸附和活化。此外,In 2表面的光生空穴诱导活性氧中心(Ti 4+ O ·– Ti 4+ OH –和 Ti 4+ O 2– Ti 4+ O ·– )O 3 /TiO 2被确定为诱导CH 4离解成·CH 3自由基的活性物质,而后者又通过·CH 3偶联成C 2 H 6。相反,在 O 2存在下,表面超氧阴离子 (O 2 – )、表面·O –自由基和 O 2 2–阴离子被确定为诱导 CH 4氧化成 CO 的活性物质。













































 京公网安备 11010802027423号
京公网安备 11010802027423号