当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Multicomponent Intermetallic Nanoparticles on Hierarchical Metal Network as Versatile Electrocatalysts for Highly Efficient Water Splitting
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2023-02-19 , DOI: 10.1002/adfm.202214412 Hang Shi 1 , Xin‐Ying Sun 1 , Yang Liu 1 , Shu‐Pei Zeng 1 , Qing‐Hua Zhang 2 , Lin Gu 3 , Tong‐Hui Wang 1 , Gao‐Feng Han 1 , Zi Wen 1 , Qian‐Rong Fang 4 , Xing‐You Lang 1 , Qing Jiang 1
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2023-02-19 , DOI: 10.1002/adfm.202214412 Hang Shi 1 , Xin‐Ying Sun 1 , Yang Liu 1 , Shu‐Pei Zeng 1 , Qing‐Hua Zhang 2 , Lin Gu 3 , Tong‐Hui Wang 1 , Gao‐Feng Han 1 , Zi Wen 1 , Qian‐Rong Fang 4 , Xing‐You Lang 1 , Qing Jiang 1
Affiliation
|
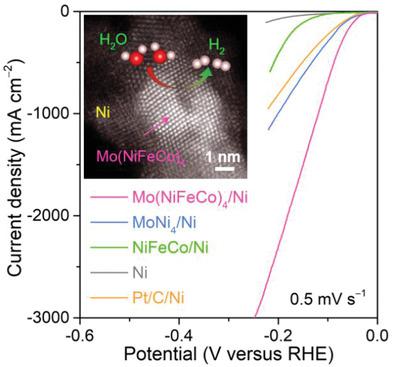
Developing high-efficiency and cost-effective alloy catalysts toward hydrogen-evolution reaction (HER) is crucial for large-scale hydrogen production via electrochemical water splitting, but conventional single-principal-element alloy design usually causes insufficient activity and durability of state-of-the-art multimetallic catalysts based on non-precious transition metals. Herein, we report multicomponent intermetallic Mo(NiFeCo)4 nanoparticles seamlessly integrated on hierarchical nickel network (Mo(NiFeCo)4/Ni) as robust hydrogen-evolution electrocatalysts with remarkably improved activity and durability by making use of iron and cobalt atoms partially substituting nickel sites to form high-entropy NiFeCo sublattice in intermetallic MoNi4 matrix, which serve as bifunctional electroactive sites for both water dissociation and adsorption/combination of hydrogen intermediate and improves thermodynamic stability. By virtue of bicontinuous nanoporous nickel skeleton facilitating electron/ion transportation, self-supported nanoporous Mo(NiFeCo)4/Ni electrode exhibits exceptional HER electrocatalysis, with low Tafel slope (≈35 mV dec−1), high current density (≈2300 mA cm−2) at low overpotential (200 mV) and long-term durability in 1 m KOH. When coupled to its electrooxidized and nitrified derivative for oxygen-evolution reaction, their alkaline water electrolyzers operate with a superior overall water-splitting output, outperforming the one constructed with commercially available noble-metal-based catalysts. These electrochemical properties make it an attractive candidate as electrocatalyst in alkaline water electrolysis for large-scale hydrogen generation.
中文翻译:

分层金属网络上的多组分金属间化合物纳米颗粒作为高效水分解的多功能电催化剂
开发用于析氢反应 (HER) 的高效且具有成本效益的合金催化剂对于通过电化学水分解进行大规模制氢至关重要,但传统的单主元素合金设计通常会导致状态的活性和耐久性不足- 基于非贵金属过渡金属的最先进的多金属催化剂。在此,我们报道了无缝集成在多级镍网络 (Mo(NiFeCo) 4 /Ni) 上的多组分金属间化合物 Mo(NiFeCo) 4纳米粒子作为稳健的析氢电催化剂,通过利用铁和钴原子部分取代镍,显着提高了活性和耐久性在金属间化合物 MoNi 4中形成高熵 NiFeCo 亚晶格的位点基质,作为氢中间体的水解离和吸附/结合的双功能电活性位点,并提高热力学稳定性。凭借促进电子/离子传输的双连续纳米多孔镍骨架,自支撑纳米多孔 Mo(NiFeCo) 4 /Ni 电极表现出优异的 HER 电催化,具有低 Tafel 斜率(≈35 mV dec -1)、高电流密度(≈2300 mA cm -2 ) 在低过电势 (200 mV) 和 1 m的长期耐久性 氢氧化钾。当与其用于析氧反应的电氧化和硝化衍生物偶联时,他们的碱性水电解槽以优异的整体水分解输出运行,优于使用市售贵金属基催化剂构建的电解槽。这些电化学特性使其成为碱性水电解中用于大规模制氢的电催化剂的有吸引力的候选者。
更新日期:2023-02-19
中文翻译:

分层金属网络上的多组分金属间化合物纳米颗粒作为高效水分解的多功能电催化剂
开发用于析氢反应 (HER) 的高效且具有成本效益的合金催化剂对于通过电化学水分解进行大规模制氢至关重要,但传统的单主元素合金设计通常会导致状态的活性和耐久性不足- 基于非贵金属过渡金属的最先进的多金属催化剂。在此,我们报道了无缝集成在多级镍网络 (Mo(NiFeCo) 4 /Ni) 上的多组分金属间化合物 Mo(NiFeCo) 4纳米粒子作为稳健的析氢电催化剂,通过利用铁和钴原子部分取代镍,显着提高了活性和耐久性在金属间化合物 MoNi 4中形成高熵 NiFeCo 亚晶格的位点基质,作为氢中间体的水解离和吸附/结合的双功能电活性位点,并提高热力学稳定性。凭借促进电子/离子传输的双连续纳米多孔镍骨架,自支撑纳米多孔 Mo(NiFeCo) 4 /Ni 电极表现出优异的 HER 电催化,具有低 Tafel 斜率(≈35 mV dec -1)、高电流密度(≈2300 mA cm -2 ) 在低过电势 (200 mV) 和 1 m的长期耐久性 氢氧化钾。当与其用于析氧反应的电氧化和硝化衍生物偶联时,他们的碱性水电解槽以优异的整体水分解输出运行,优于使用市售贵金属基催化剂构建的电解槽。这些电化学特性使其成为碱性水电解中用于大规模制氢的电催化剂的有吸引力的候选者。































 京公网安备 11010802027423号
京公网安备 11010802027423号