Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2023-02-17 , DOI: 10.1016/j.molliq.2023.121480 Wenchao Dong , Runqing Liu , Changtao Wang , Xianwen Zhu , Zhenhui Xie , Wei Sun
|
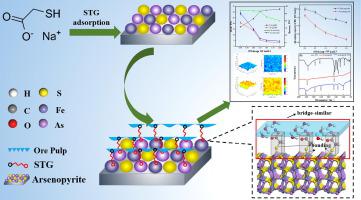
毒砂是铜精矿中存在的一种有害物质,在冶炼过程中造成严重的环境污染。然而,毒砂的选择性抑制仍然是浮选的挑战。本研究旨在分析硫代甘酸钠(STG)在Cu-As分离浮选中对毒砂的抑制机理。微浮选试验证实,STG 能够在黄铜矿浮选中选择性地抑制毒砂并降低铜精矿中的砷含量。局域电化学阻抗谱 (LEIS) 表明 STG 更倾向于吸附在毒砂上而不是黄铜矿上,这增强了电化学阻抗并降低了表面反应性。此外,表面吸附试验和接触角测量表明,STG阻碍了黄药丁酯(BX)在毒砂表面的吸附,从而进一步增强了毒砂的润湿性。傅里叶变换红外光谱(FTIR)、DFT计算和分子动力学模拟(MDS)结果表明,STG通过其-SH基团与毒砂表面的Fe和As位点发生化学键合,并与水分子形成氢键通过分子顶部的 -COO- 基团。最终,在毒砂、STG 和水分子之间产生了类似桥的结构。这种结构导致毒砂表面覆盖了一层稳定的亲水膜,因此其可浮性严重恶化。傅里叶变换红外光谱(FTIR)、DFT计算和分子动力学模拟(MDS)结果表明,STG通过其-SH基团与毒砂表面的Fe和As位点发生化学键合,并与水分子形成氢键通过分子顶部的 -COO- 基团。最终,在毒砂、STG 和水分子之间产生了类似桥的结构。这种结构导致毒砂表面覆盖了一层稳定的亲水膜,因此其可浮性严重恶化。傅里叶变换红外光谱(FTIR)、DFT计算和分子动力学模拟(MDS)结果表明,STG通过其-SH基团与毒砂表面的Fe和As位点发生化学键合,并与水分子形成氢键通过分子顶部的 -COO- 基团。最终,在毒砂、STG 和水分子之间产生了类似桥的结构。这种结构导致毒砂表面覆盖了一层稳定的亲水膜,因此其可浮性严重恶化。它通过分子顶部的-COO-基团与水分子形成氢键。最终,在毒砂、STG 和水分子之间产生了类似桥的结构。这种结构导致毒砂表面覆盖了一层稳定的亲水膜,因此其可浮性严重恶化。它通过分子顶部的-COO-基团与水分子形成氢键。最终,在毒砂、STG 和水分子之间产生了类似桥的结构。这种结构导致毒砂表面覆盖了一层稳定的亲水膜,因此其可浮性严重恶化。

"点击查看英文标题和摘要"






























 京公网安备 11010802027423号
京公网安备 11010802027423号