Journal of Colloid and Interface Science ( IF 9.4 ) Pub Date : 2023-02-09 , DOI: 10.1016/j.jcis.2023.02.027 Yanyan Li 1 , Guangyao Dang 1 , Muhammad Rizwan Younis 2 , Yutao Cao 1 , Kaiqi Wang 1 , Xiao Sun 1 , Wenxian Zhang 1 , Xianwen Zou 1 , Hui Shen 1 , Ruibing An 1 , Lifeng Dong 1 , Jian Dong 1
|
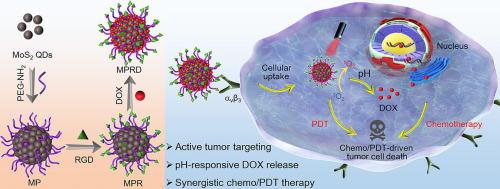
The combination of imaging and different therapeutic strategies into one single nanoplatform often demonstrates improved efficacy over monotherapy in cancer treatments. Herein, a multifunctional nanoplatform (labelled as MPRD) based on molybdenum disulfide quantum dots (MoS2 QDs) is developed to achieve enhanced antitumor efficiency by integrating fluorescence imaging, tumor-targeting and synergistic chemo/photodynamic therapy (PDT) into one system. First, polyethylene glycol (PEG)ylated MoS2 QDs (MP) with desirable stability are synthesized via a hydrothermal process using MoS2 QDs and carboxyamino-terminated oligomeric PEG as raw materials. Then, MP were conjugated with arginine-glycine-aspartic acid (RGD) peptide via amidation to form a novel nanocarrier (MPR), which possesses strong blue fluorescence, good biocompatibility and ανβ3 receptor-mediated targeting ability. More importantly, MPR generated reactive oxygen species under 808 nm laser activation to realize targeted antitumor PDT. Further doxorubicin (DOX) was loaded onto MPR, which endows MPRD with localized chemotherapy and pH-responsive drug release. The MPRD exhibits improved chemotherapy performance on HepG2 cells (overexpressing integrin ανβ3) owing to enhanced cellular uptake mediated by ανβ3 receptor and effective drug release triggered by intracellular pH. Notably, MPRD with efficient tumor targeting ability and high chemo/PDT efficacy under NIR laser irradiation is capable of inhibiting HepG2 tumor cell growth both in vitro and in vivo, which is significantly superior to each individual therapy. These findings demonstrate that MPRD holds great potential in effective cancer therapy.
中文翻译:

肽功能化的主动靶向 MoS2 纳米球用于荧光成像引导的可控 pH 响应药物递送和协作化学/光动力疗法
将成像和不同的治疗策略组合到一个单一的纳米平台中,通常证明在癌症治疗中比单一疗法具有更高的疗效。在此,开发了一种基于二硫化钼量子点 (MoS 2 QD) 的多功能纳米平台(标记为 MPRD),通过将荧光成像、肿瘤靶向和协同化学/光动力疗法 (PDT) 集成到一个系统中来提高抗肿瘤效率。首先,使用 MoS 2通过水热法合成具有理想稳定性的聚乙二醇 (PEG) 化的 MoS 2 QD (MP)以量子点和羧基氨基封端的低聚聚乙二醇为原料。然后,MP通过酰胺化与精氨酸-甘氨酸-天冬氨酸(RGD)肽偶联形成新型纳米载体(MPR),具有强蓝色荧光、良好的生物相容性和α ν β 3 受体介导的靶向能力。更重要的是,MPR 在 808 nm 激光激活下产生活性氧,实现靶向抗肿瘤 PDT。进一步将多柔比星 (DOX) 加载到 MPR 上,赋予 MPRD 局部化疗和 pH 响应药物释放。由于 α ν β 3介导的细胞摄取增强,MPRD 对 HepG2 细胞(过表达整合素 α ν β 3 )表现出改善的化疗性能受体和细胞内 pH 触发的有效药物释放。值得注意的是,MPRD 在 NIR 激光照射下具有高效的肿瘤靶向能力和高化疗/PDT 疗效,能够在体外和体内抑制 HepG2 肿瘤细胞的生长,显着优于单独治疗。这些发现表明 MPRD 在有效的癌症治疗中具有巨大潜力。































 京公网安备 11010802027423号
京公网安备 11010802027423号