Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2023-02-18 , DOI: 10.1016/j.bmcl.2023.129189 Andrei V Erkin 1 , Evgeny B Serebryakov 2 , Viktor I Krutikov 1
|
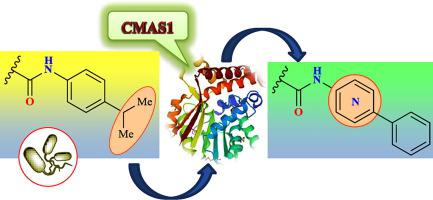
The synthesis of 2-[(2-amino-6-methylpyrimidin-4-yl)sulfanyl]-N-arylacetamides 6a-j was encouraged by their antibacterial activity and drug-likeness predictions. Of the compounds, two bearing 4-isopropylphenyl 6c and 2,5-dichlorophenyl 6i moieties were found to be threefold more potent than the first-line tuberculosis drug ethambutol. A molecular docking study revealed that compound 6c may selectively bind to cyclopropane mycolic acid synthase 1, an enzyme essential for the construction of the tuberculosis bacteria cell wall. Keeping this in mind, a recently developed ligand-based virtual screening strategy combining the molecular similarity search and docking approaches was adopted to identify more potent analogs of the parent compound. As a result, a series of new ligands 18p-w with phenyl-substituted azinyl amide groups were in silico discovered. Due to their high binding affinities to the enzyme and improved toxicity profiles, the ligands are undoubtedly worth future synthetic efforts.
中文翻译:

2-[(2-Amino-6-methylpyrimidin-4-yl)sulfanyl]-N-arylacetamides:一类新型抗结核药物的发现及其进一步结构修饰的前景
2-[(2-amino-6-methylpyrimidin-4-yl)sulfanyl]-N-arylacetamides 6a-j的合成因其抗菌活性和类药性预测而受到鼓舞。在这些化合物中,两个带有 4-异丙基苯基6c和 2,5-二氯苯基6i部分的化合物被发现比一线结核病药物乙胺丁醇强三倍。分子对接研究表明,化合物6c可以选择性地结合环丙烷分支菌酸合酶 1,这是一种构建结核菌细胞壁所必需的酶。牢记这一点,采用了最近开发的基于配体的虚拟筛选策略,结合了分子相似性搜索和对接方法,以识别更有效的母体化合物类似物。结果,在计算机中发现了一系列具有苯基取代的吖嗪基酰胺基团的新配体18p-w 。由于它们对酶的高结合亲和力和改进的毒性特征,配体无疑值得未来的合成努力。






























 京公网安备 11010802027423号
京公网安备 11010802027423号