当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic Insight into Palladium/Brønsted Acid Catalyzed Methoxycarbonylation and Hydromethoxylation of Internal Alkene: A Computational Study
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2023-02-17 , DOI: 10.1021/acs.inorgchem.2c04291 Lingli Han 1, 2 , Kang Lv 1 , Teng Wang 2 , Zitong Meng 2 , Jing Zhang 1, 2 , Tao Liu 1, 2
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2023-02-17 , DOI: 10.1021/acs.inorgchem.2c04291 Lingli Han 1, 2 , Kang Lv 1 , Teng Wang 2 , Zitong Meng 2 , Jing Zhang 1, 2 , Tao Liu 1, 2
Affiliation
|
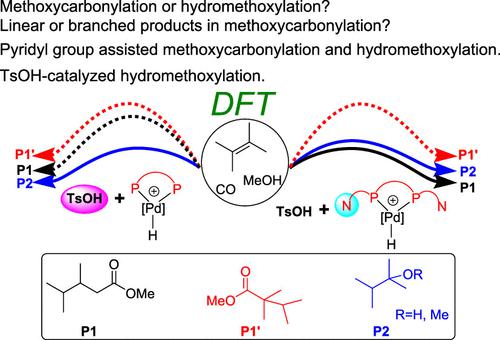
Density functional theory (DFT) calculations were performed to study the palladium/Brønsted acid-catalyzed methoxycarbonylation and hydromethoxylation reactions of internal alkene. The calculated results show that the pyridyl group (N atom) in bidentate phosphine ligand with built-in base (L1) plays a crucial role in controlling the selectivity. With the help of the pyridyl group, the methanolysis steps in the methoxycarbonylation reaction and the hydromethoxylation reaction become easy, and both the linear ester methyl 3,4-dimethylpentanoate (P1) and the hydromethoxylation product 2-methoxy-2,3-dimethylbutane (P2) could be obtained. In contrast, the possibility of leading to branched ester P1′ was ruled out according to our calculations. The steric effect could account for the observed selectivity. In the presence of the DPEphos ligand (L2) that does not bear the pyridyl group, the methanolysis step in the methoxycarbonylation reaction becomes the rate-determining step with a high overall energy barrier. Neither linear nor branched methoxycarbonylation product could be generated. The palladium/Brønsted acid co-catalyzed hydromethoxylation also become difficult without the assistance of the pyridyl group in the presence of the L2 ligand. Instead, TsOH-catalyzed hydromethoxylation reaction could take place to generate the ether product P2.
中文翻译:

钯/布朗斯台德酸催化的内烯烃甲氧基羰基化和氢甲氧基化的机理洞察:计算研究
进行密度泛函理论 (DFT) 计算以研究钯/Brønsted 酸催化的内烯烃的甲氧基羰基化和氢化甲氧基化反应。计算结果表明,内置碱基( L1 )的双齿膦配体中的吡啶基(N原子)对选择性的控制起着至关重要的作用。在吡啶基的帮助下,甲氧基羰基化反应和加氢甲氧基化反应中的甲醇分解步骤变得容易,直链酯3,4-二甲基戊酸甲酯(P1)和加氢甲氧基化产物2-甲氧基-2,3-二甲基丁烷(P2 ) 可以得到。相反,导致支化酯P1'的可能性根据我们的计算排除了。空间效应可以解释观察到的选择性。在不带有吡啶基的DPEphos配体( L2 )存在下,甲氧基羰基化反应中的甲醇分解步骤成为具有高总能垒的决速步骤。既不能产生直链也不能产生支链的甲氧基羰基化产物。在L2配体存在的情况下,如果没有吡啶基的帮助,钯/布朗斯台德酸共催化的氢化甲氧基化也变得困难。相反,可以发生 TsOH 催化的加氢甲氧基化反应,生成醚产物P2。
更新日期:2023-02-17
中文翻译:

钯/布朗斯台德酸催化的内烯烃甲氧基羰基化和氢甲氧基化的机理洞察:计算研究
进行密度泛函理论 (DFT) 计算以研究钯/Brønsted 酸催化的内烯烃的甲氧基羰基化和氢化甲氧基化反应。计算结果表明,内置碱基( L1 )的双齿膦配体中的吡啶基(N原子)对选择性的控制起着至关重要的作用。在吡啶基的帮助下,甲氧基羰基化反应和加氢甲氧基化反应中的甲醇分解步骤变得容易,直链酯3,4-二甲基戊酸甲酯(P1)和加氢甲氧基化产物2-甲氧基-2,3-二甲基丁烷(P2 ) 可以得到。相反,导致支化酯P1'的可能性根据我们的计算排除了。空间效应可以解释观察到的选择性。在不带有吡啶基的DPEphos配体( L2 )存在下,甲氧基羰基化反应中的甲醇分解步骤成为具有高总能垒的决速步骤。既不能产生直链也不能产生支链的甲氧基羰基化产物。在L2配体存在的情况下,如果没有吡啶基的帮助,钯/布朗斯台德酸共催化的氢化甲氧基化也变得困难。相反,可以发生 TsOH 催化的加氢甲氧基化反应,生成醚产物P2。































 京公网安备 11010802027423号
京公网安备 11010802027423号