当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cr-Catalyzed Regio-, Diastereo-, and Enantioselective Reductive Couplings of Ketones and Propargyl Halides
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-02-17 , DOI: 10.1021/acscatal.3c00177 Xiaochong Guo 1, 2 , Zhaoxin Shi 2 , Feng-Hua Zhang 2, 3 , Zhaobin Wang 2, 3
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-02-17 , DOI: 10.1021/acscatal.3c00177 Xiaochong Guo 1, 2 , Zhaoxin Shi 2 , Feng-Hua Zhang 2, 3 , Zhaobin Wang 2, 3
Affiliation
|
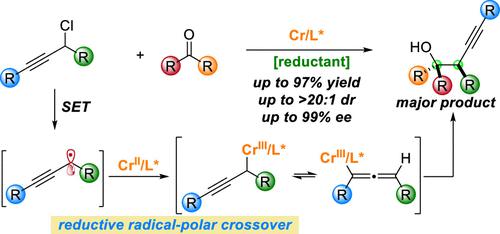
Enantioconvergent reductive couplings of racemic alkyl halides with carbonyl compounds provide efficient access to valuable chiral alcohols, especially those bearing vicinal stereocenters. However, limited success has been achieved due to the challenging reactivity and stereoselectivity control. Herein, we developed the Cr-catalyzed asymmetric reductive coupling of racemic propargylic chlorides and ketones, affording valuable chiral tertiary alcohols bearing vicinal stereocenters. These reactions proceed efficiently under mild conditions in a radical–polar crossover manner with good regio-, diastereo-, and enantioselectivity control. Preliminary mechanistic studies, including radical trapping, nonlinear effect, and UV–vis spectroscopy, provide insights into the radical-involved catalytic cycle. DFT calculations suggest that the regio- and stereoselectivity are determined by the Zimmerman–Traxler-type ketone addition transition states under Curtin–Hammett conditions.
中文翻译:

Cr 催化的酮和炔丙基卤化物的区域、非对映和对映选择性还原偶联
外消旋烷基卤化物与羰基化合物的对映收敛还原偶联提供了获得有价值的手性醇的有效途径,尤其是那些带有邻位立构中心的手性醇。然而,由于具有挑战性的反应性和立体选择性控制,取得的成功有限。在此,我们开发了 Cr 催化的外消旋炔丙基氯和酮的不对称还原偶联,提供了具有邻位立构中心的有价值的手性叔醇。这些反应在温和条件下以自由基-极性交叉方式有效进行,具有良好的区域选择性、非对映选择性和对映选择性控制。初步的机理研究,包括自由基捕获、非线性效应和紫外-可见光谱,提供了对自由基参与的催化循环的见解。
更新日期:2023-02-17
中文翻译:

Cr 催化的酮和炔丙基卤化物的区域、非对映和对映选择性还原偶联
外消旋烷基卤化物与羰基化合物的对映收敛还原偶联提供了获得有价值的手性醇的有效途径,尤其是那些带有邻位立构中心的手性醇。然而,由于具有挑战性的反应性和立体选择性控制,取得的成功有限。在此,我们开发了 Cr 催化的外消旋炔丙基氯和酮的不对称还原偶联,提供了具有邻位立构中心的有价值的手性叔醇。这些反应在温和条件下以自由基-极性交叉方式有效进行,具有良好的区域选择性、非对映选择性和对映选择性控制。初步的机理研究,包括自由基捕获、非线性效应和紫外-可见光谱,提供了对自由基参与的催化循环的见解。

































 京公网安备 11010802027423号
京公网安备 11010802027423号