当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photoredox-Nickel Dual-Catalyzed C-Alkylation of Secondary Nitroalkanes: Access to Sterically Hindered α-Tertiary Amines
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-02-16 , DOI: 10.1021/jacs.2c13174 Sina Rezazadeh 1 , Maxwell I Martin 1 , Raphael S Kim 1 , Glenn P A Yap 1 , Joel Rosenthal 1 , Donald A Watson 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-02-16 , DOI: 10.1021/jacs.2c13174 Sina Rezazadeh 1 , Maxwell I Martin 1 , Raphael S Kim 1 , Glenn P A Yap 1 , Joel Rosenthal 1 , Donald A Watson 1
Affiliation
|
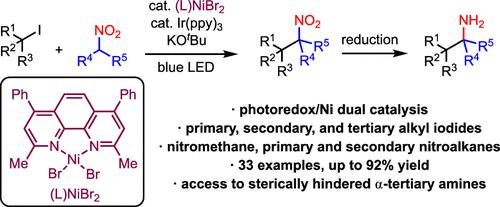
The preparation of tertiary nitroalkanes via the nickel-catalyzed alkylation of secondary nitroalkanes using aliphatic iodides is reported. Previously, catalytic access to this important class of nitroalkanes via alkylation has not been possible due to the inability of catalysts to overcome the steric demands of the products. However, we have now found that the use of a nickel catalyst in combination with a photoredox catalyst and light leads to much more active alkylation catalysts. These can now access tertiary nitroalkanes. The conditions are scalable as well as air and moisture tolerant. Importantly, reduction of the tertiary nitroalkane products allows rapid access to α-tertiary amines.
中文翻译:

光氧化还原-镍双催化仲硝基烷烃的 C-烷基化:获得空间位阻 α-叔胺
据报道,通过使用脂肪族碘化物对仲硝基烷烃进行镍催化烷基化来制备叔硝基烷烃。此前,由于催化剂无法克服产物的空间需求,通过烷基化催化获得这一类重要的硝基烷烃是不可能的。然而,我们现在发现,将镍催化剂与光氧化还原催化剂和光结合使用会产生活性更高的烷基化催化剂。这些现在可以接触叔硝基烷烃。这些条件是可扩展的以及耐空气和耐湿气的。重要的是,叔硝基烷产物的还原可以快速获得α-叔胺。
更新日期:2023-02-16
中文翻译:

光氧化还原-镍双催化仲硝基烷烃的 C-烷基化:获得空间位阻 α-叔胺
据报道,通过使用脂肪族碘化物对仲硝基烷烃进行镍催化烷基化来制备叔硝基烷烃。此前,由于催化剂无法克服产物的空间需求,通过烷基化催化获得这一类重要的硝基烷烃是不可能的。然而,我们现在发现,将镍催化剂与光氧化还原催化剂和光结合使用会产生活性更高的烷基化催化剂。这些现在可以接触叔硝基烷烃。这些条件是可扩展的以及耐空气和耐湿气的。重要的是,叔硝基烷产物的还原可以快速获得α-叔胺。






























 京公网安备 11010802027423号
京公网安备 11010802027423号