当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Differences in Molecular Adsorption Emanating from the (2 × 1) Reconstruction of Calcite(104)
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2023-02-16 , DOI: 10.1021/acs.jpclett.2c03243 Jonas Heggemann 1 , Yashasvi S Ranawat 2 , Ondřej Krejčí 2 , Adam S Foster 2, 3 , Philipp Rahe 1
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2023-02-16 , DOI: 10.1021/acs.jpclett.2c03243 Jonas Heggemann 1 , Yashasvi S Ranawat 2 , Ondřej Krejčí 2 , Adam S Foster 2, 3 , Philipp Rahe 1
Affiliation
|
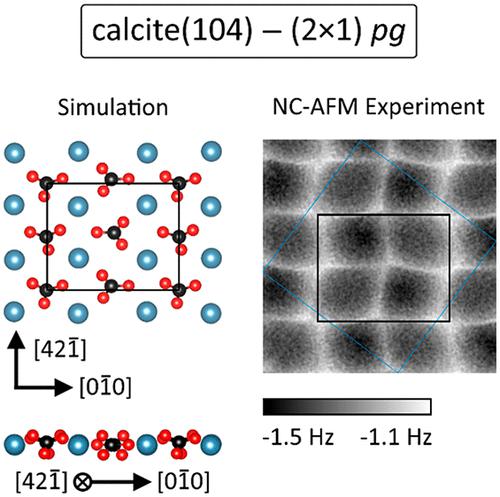
Calcite, in the natural environment the most stable polymorph of calcium carbonate (CaCO3), not only is an abundant mineral in the Earth’s crust but also forms a central constituent in the biominerals of living organisms. Intensive studies of calcite(104), the surface supporting virtually all processes, have been performed, and the interaction with a plethora of adsorbed species has been studied. Surprisingly, there is still serious ambiguity regarding the properties of the calcite(104) surface: effects such as a row-pairing or a (2 × 1) reconstruction have been reported, yet so far without physicochemical explanation. Here, we unravel the microscopic geometry of calcite(104) using high-resolution atomic force microscopy (AFM) data acquired at 5 K combined with density functional theory (DFT) and AFM image calculations. A (2 × 1) reconstruction of a pg-symmetric surface is found to be the thermodynamically most stable form. Most importantly, a decisive impact of the (2 × 1) reconstruction on adsorbed species is revealed for carbon monoxide.
中文翻译:

方解石 (2 × 1) 重建产生的分子吸附差异 (104)
方解石,自然环境中最稳定的碳酸钙多晶型物(CaCO 3), 不仅是地壳中丰富的矿物质,而且也是生物体生物矿物质的核心成分。方解石 (104) 的表面支持几乎所有过程,已经进行了深入研究,并且已经研究了与过多吸附物质的相互作用。令人惊讶的是,关于方解石 (104) 表面的性质仍然存在严重的歧义:已经报道了行配对或 (2 × 1) 重建等效应,但到目前为止还没有物理化学解释。在这里,我们使用在 5 K 下获取的高分辨率原子力显微镜 (AFM) 数据结合密度泛函理论 (DFT) 和 AFM 图像计算来揭示方解石 (104) 的微观几何结构。一个pg的 (2 × 1) 重构-对称表面被发现是热力学上最稳定的形式。最重要的是,揭示了 (2 × 1) 重建对一氧化碳吸附物质的决定性影响。
更新日期:2023-02-16
中文翻译:

方解石 (2 × 1) 重建产生的分子吸附差异 (104)
方解石,自然环境中最稳定的碳酸钙多晶型物(CaCO 3), 不仅是地壳中丰富的矿物质,而且也是生物体生物矿物质的核心成分。方解石 (104) 的表面支持几乎所有过程,已经进行了深入研究,并且已经研究了与过多吸附物质的相互作用。令人惊讶的是,关于方解石 (104) 表面的性质仍然存在严重的歧义:已经报道了行配对或 (2 × 1) 重建等效应,但到目前为止还没有物理化学解释。在这里,我们使用在 5 K 下获取的高分辨率原子力显微镜 (AFM) 数据结合密度泛函理论 (DFT) 和 AFM 图像计算来揭示方解石 (104) 的微观几何结构。一个pg的 (2 × 1) 重构-对称表面被发现是热力学上最稳定的形式。最重要的是,揭示了 (2 × 1) 重建对一氧化碳吸附物质的决定性影响。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号