当前位置:
X-MOL 学术
›
Adv. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Multivalent Nanobody Conjugate with Rigid, Reactive Oxygen Species Scavenging Scaffold for Multi-Target Therapy of Alzheimer's Disease
Advanced Materials ( IF 27.4 ) Pub Date : 2023-02-14 , DOI: 10.1002/adma.202210879 Liyuan Zhao 1, 2 , Fanling Meng 1, 2, 3 , Yingjie Li 4 , Sujuan Liu 1 , Mengmeng Xu 4 , Fan Chu 4 , Chuanzhou Li 4 , Xiangliang Yang 1, 2 , Liang Luo 1, 2, 3
Advanced Materials ( IF 27.4 ) Pub Date : 2023-02-14 , DOI: 10.1002/adma.202210879 Liyuan Zhao 1, 2 , Fanling Meng 1, 2, 3 , Yingjie Li 4 , Sujuan Liu 1 , Mengmeng Xu 4 , Fan Chu 4 , Chuanzhou Li 4 , Xiangliang Yang 1, 2 , Liang Luo 1, 2, 3
Affiliation
|
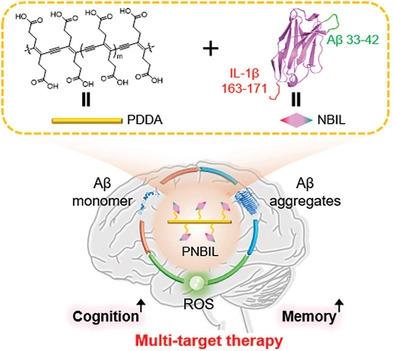
Efficient therapeutic strategies that concurrently target both Aβ aggregation and oxidative stress in the Alzheimer's disease (AD) microenvironment emerge as a cutting-edge tool to combat the intricate pathogenesis of AD. Here, a multivalent nanobody conjugate with rigid, reactive oxygen species (ROS) scavenging scaffold is developed to achieve simultaneous Aβ amyloidogenesis mitigation, ROS elimination, and Aβ plaque clearance. Grafting Aβ segment (33-GLMVGGVVIA-42) into the third complementary-determining region of a parent nanobody generates an engineered nanobody NB that can recognize Aβ and inhibit its aggregation through homotypic interactions. NB is further genetically modified with a fragment of human interleukin-1β (163-VQGEESNDK-171), so that the obtained fusion nanobody NBIL can also facilitate the Aβ clearance by microglia. Linking NBIL covalently onto a rigid, ROS scavenging scaffold poly(deca-4,6-diynedioic acid) (PDDA) creates the multivalent nanobody conjugate PNBIL, which not only boosts the binding affinity between NBIL and Aβ aggregates for nearly 100 times but also possesses a long-term capability of oxidative stress alleviation, inflammation reduction, and neuron protection. PNBIL has significantly attenuated symptoms on two AD mouse models through amyloidogenesis inhibition and AD microenvironment modulation, validating that the multivalent nanobody conjugate design based on combinatory nanobody and molecular engineering is a promising approach of multi-target therapeutic strategies.
中文翻译:

多价纳米抗体结合刚性活性氧清除支架用于阿尔茨海默病的多靶点治疗
同时针对阿尔茨海默病 (AD) 微环境中的 Aβ 聚集和氧化应激的有效治疗策略成为对抗 AD 复杂发病机制的尖端工具。在这里,开发了一种具有刚性活性氧 (ROS) 清除支架的多价纳米抗体缀合物,以同时实现 Aβ 淀粉样变性缓解、ROS 消除和 Aβ 斑块清除。将 Aβ 片段 (33-GLMVGGVVIA-42) 移植到母体纳米抗体的第三个互补决定区中,会生成一个工程化的纳米抗体 NB,它可以识别 Aβ 并通过同型相互作用抑制其聚集。NB进一步用人白细胞介素-1β片段(163-VQGEESNDK-171)进行基因修饰,使得获得的融合纳米抗体NBIL也可以促进小胶质细胞对Aβ的清除。将 NBIL 共价连接到刚性 ROS 清除支架聚(deca-4,6-二炔二酸)(PDDA) 上产生多价纳米抗体偶联物 PNBIL,它不仅将 NBIL 和 Aβ 聚集体之间的结合亲和力提高了近 100 倍,而且还具有长期的氧化应激缓解、炎症减少和神经元保护能力。PNBIL 通过淀粉样蛋白生成抑制和 AD 微环境调节显着减轻了两种 AD 小鼠模型的症状,验证了基于组合纳米抗体和分子工程的多价纳米抗体偶联设计是一种有前途的多靶点治疗策略。它不仅将 NBIL 与 Aβ 聚集体的结合亲和力提高了近 100 倍,而且还具有长期的氧化应激缓解、炎症减少和神经元保护能力。PNBIL 通过淀粉样蛋白生成抑制和 AD 微环境调节显着减轻了两种 AD 小鼠模型的症状,验证了基于组合纳米抗体和分子工程的多价纳米抗体偶联设计是一种有前途的多靶点治疗策略。它不仅将 NBIL 与 Aβ 聚集体的结合亲和力提高了近 100 倍,而且还具有长期的氧化应激缓解、炎症减少和神经元保护能力。PNBIL 通过淀粉样蛋白生成抑制和 AD 微环境调节显着减轻了两种 AD 小鼠模型的症状,验证了基于组合纳米抗体和分子工程的多价纳米抗体偶联设计是一种有前途的多靶点治疗策略。
更新日期:2023-02-14
中文翻译:

多价纳米抗体结合刚性活性氧清除支架用于阿尔茨海默病的多靶点治疗
同时针对阿尔茨海默病 (AD) 微环境中的 Aβ 聚集和氧化应激的有效治疗策略成为对抗 AD 复杂发病机制的尖端工具。在这里,开发了一种具有刚性活性氧 (ROS) 清除支架的多价纳米抗体缀合物,以同时实现 Aβ 淀粉样变性缓解、ROS 消除和 Aβ 斑块清除。将 Aβ 片段 (33-GLMVGGVVIA-42) 移植到母体纳米抗体的第三个互补决定区中,会生成一个工程化的纳米抗体 NB,它可以识别 Aβ 并通过同型相互作用抑制其聚集。NB进一步用人白细胞介素-1β片段(163-VQGEESNDK-171)进行基因修饰,使得获得的融合纳米抗体NBIL也可以促进小胶质细胞对Aβ的清除。将 NBIL 共价连接到刚性 ROS 清除支架聚(deca-4,6-二炔二酸)(PDDA) 上产生多价纳米抗体偶联物 PNBIL,它不仅将 NBIL 和 Aβ 聚集体之间的结合亲和力提高了近 100 倍,而且还具有长期的氧化应激缓解、炎症减少和神经元保护能力。PNBIL 通过淀粉样蛋白生成抑制和 AD 微环境调节显着减轻了两种 AD 小鼠模型的症状,验证了基于组合纳米抗体和分子工程的多价纳米抗体偶联设计是一种有前途的多靶点治疗策略。它不仅将 NBIL 与 Aβ 聚集体的结合亲和力提高了近 100 倍,而且还具有长期的氧化应激缓解、炎症减少和神经元保护能力。PNBIL 通过淀粉样蛋白生成抑制和 AD 微环境调节显着减轻了两种 AD 小鼠模型的症状,验证了基于组合纳米抗体和分子工程的多价纳米抗体偶联设计是一种有前途的多靶点治疗策略。它不仅将 NBIL 与 Aβ 聚集体的结合亲和力提高了近 100 倍,而且还具有长期的氧化应激缓解、炎症减少和神经元保护能力。PNBIL 通过淀粉样蛋白生成抑制和 AD 微环境调节显着减轻了两种 AD 小鼠模型的症状,验证了基于组合纳米抗体和分子工程的多价纳米抗体偶联设计是一种有前途的多靶点治疗策略。































 京公网安备 11010802027423号
京公网安备 11010802027423号