当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photocatalytic C–N coupling towards urea synthesis with a palladium-supported CeO2 catalyst
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2023-02-13 , DOI: 10.1039/d2cy02086f
Shuyi Yang 1 , Jiayi Deng 1 , Jiaying Chen 1 , Qingmei Tan 1 , Tianren Liu 1 , Ke Chen 1 , Dongxue Han 1, 2 , Yingming Ma 1 , Mengjiao Dai 3 , Li Niu 1
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2023-02-13 , DOI: 10.1039/d2cy02086f
Shuyi Yang 1 , Jiayi Deng 1 , Jiaying Chen 1 , Qingmei Tan 1 , Tianren Liu 1 , Ke Chen 1 , Dongxue Han 1, 2 , Yingming Ma 1 , Mengjiao Dai 3 , Li Niu 1
Affiliation
|
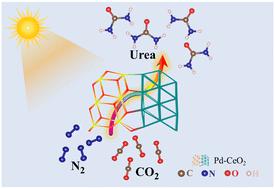
The conversion of N2 and CO2 into urea through a photocatalytic C–N coupling reaction under ambient conditions serves as a novel green avenue for urea synthesis. However, the poor adsorption and C–N coupling capability of inert gas molecules hinder the efficient catalytic activity. Herein, palladium-decorated CeO2 (Pd–CeO2) was demonstrated as an efficient photocatalyst for C–N coupling reaction and delivered a remarkable urea yield rate of 9.2 μmol h−1 g−1, which was superior to that of pristine CeO2 (2.5 μmol h−1 g−1). Comprehensive investigations further endorsed that the emerged space-charge region in the CeO2(111)/Pd(111) interface not only effectively facilitates the targeted capture and activation of inert CO2 and N2 but also stabilizes the formation of key intermediates (*NCON). Besides, the effective inhibition of the endothermic *NNH intermediate is conducive to the subsequent C–N coupling process and the improvement of reaction selectivity. The reaction mechanism was studied in detail by density functional theory (DFT) with the formation of a C–N bond via a thermodynamically spontaneous reaction between *N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N* and CO. This work provides novel insights into the conversion of CO2 and N2 into urea.
N* and CO. This work provides novel insights into the conversion of CO2 and N2 into urea.
中文翻译:

用钯负载的 CeO2 催化剂光催化 C-N 偶联合成尿素
在环境条件下通过光催化 C-N 偶联反应将 N 2和 CO 2转化为尿素是尿素合成的新型绿色途径。然而,惰性气体分子较差的吸附和C-N偶联能力阻碍了高效的催化活性。在此,钯装饰的 CeO 2 (Pd–CeO 2 ) 被证明是一种有效的 C–N 偶联反应光催化剂,并提供了 9.2 μmol h −1 g −1的显着尿素产率,优于原始 CeO 的产率2 (2.5 μmol·h −1 g −1). 综合调查进一步证实,CeO 2 (111)/Pd(111) 界面中出现的空间电荷区不仅有效地促进了惰性 CO 2和 N 2的靶向捕获和活化,而且稳定了关键中间体的形成 (* NCON)。此外,有效抑制吸热的*NNH中间体有利于后续的C-N偶联过程和反应选择性的提高。通过*N![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N* 和 CO之间的热力学自发反应形成 C-N 键,通过密度泛函理论 (DFT) 详细研究了反应机理。这项工作为 CO 2和 N 2的转化提供了新的见解变成尿素。
N* 和 CO之间的热力学自发反应形成 C-N 键,通过密度泛函理论 (DFT) 详细研究了反应机理。这项工作为 CO 2和 N 2的转化提供了新的见解变成尿素。
更新日期:2023-02-13
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N* and CO. This work provides novel insights into the conversion of CO2 and N2 into urea.
N* and CO. This work provides novel insights into the conversion of CO2 and N2 into urea.
中文翻译:

用钯负载的 CeO2 催化剂光催化 C-N 偶联合成尿素
在环境条件下通过光催化 C-N 偶联反应将 N 2和 CO 2转化为尿素是尿素合成的新型绿色途径。然而,惰性气体分子较差的吸附和C-N偶联能力阻碍了高效的催化活性。在此,钯装饰的 CeO 2 (Pd–CeO 2 ) 被证明是一种有效的 C–N 偶联反应光催化剂,并提供了 9.2 μmol h −1 g −1的显着尿素产率,优于原始 CeO 的产率2 (2.5 μmol·h −1 g −1). 综合调查进一步证实,CeO 2 (111)/Pd(111) 界面中出现的空间电荷区不仅有效地促进了惰性 CO 2和 N 2的靶向捕获和活化,而且稳定了关键中间体的形成 (* NCON)。此外,有效抑制吸热的*NNH中间体有利于后续的C-N偶联过程和反应选择性的提高。通过*N
![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N* 和 CO之间的热力学自发反应形成 C-N 键,通过密度泛函理论 (DFT) 详细研究了反应机理。这项工作为 CO 2和 N 2的转化提供了新的见解变成尿素。
N* 和 CO之间的热力学自发反应形成 C-N 键,通过密度泛函理论 (DFT) 详细研究了反应机理。这项工作为 CO 2和 N 2的转化提供了新的见解变成尿素。

































 京公网安备 11010802027423号
京公网安备 11010802027423号