当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Homologation of the Alkyl Side Chain of Antimitotic Phenyl 4-(2-Oxo-3-alkylimidazolidin-1-yl)benzenesulfonate Prodrugs Selectively Targeting CYP1A1-Expressing Breast Cancers Improves Their Stability in Rodent Liver Microsomes
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2023-02-13 , DOI: 10.1021/acs.jmedchem.2c01268 Atziri Corin Chavez Alvarez 1, 2, 3 , Chahrazed Bouzriba 1, 2 , Emmanuel Moreau 4, 5 , Philippe Auzeloux 4, 5 , Sophie Besse 4, 5 , Vincent Ouellette 1, 2 , Mitra Zarifi Khosroshahi 1, 2 , Marie-France Côté 2 , Sylvie Pilote 3 , Elisabeth Miot-Noirault 4, 5 , Jean-Michel Chezal 4, 5 , Chantale Simard 1, 3 , René C-Gaudreault 2, 6 , Sébastien Fortin 1, 2
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2023-02-13 , DOI: 10.1021/acs.jmedchem.2c01268 Atziri Corin Chavez Alvarez 1, 2, 3 , Chahrazed Bouzriba 1, 2 , Emmanuel Moreau 4, 5 , Philippe Auzeloux 4, 5 , Sophie Besse 4, 5 , Vincent Ouellette 1, 2 , Mitra Zarifi Khosroshahi 1, 2 , Marie-France Côté 2 , Sylvie Pilote 3 , Elisabeth Miot-Noirault 4, 5 , Jean-Michel Chezal 4, 5 , Chantale Simard 1, 3 , René C-Gaudreault 2, 6 , Sébastien Fortin 1, 2
Affiliation
|
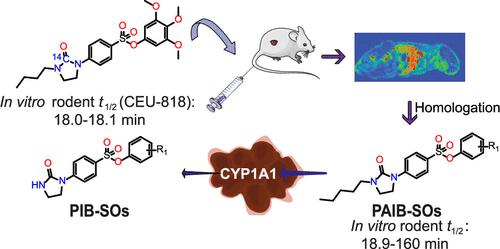
Phenyl 4-(2-oxo-3-alkylimidazolidin-1-yl)benzenesulfonates (PAIB-SOs) are a new family of antimitotic prodrugs bioactivated in breast cancer cells expressing CYP1A1. In this study, we report that the 14C-labeled prototypical PAIB-SO [14C]CEU-818 and its antimitotic counterpart [14C]CEU-602 are distributed in whole mouse body and they show a short half-life in mice. To circumvent this limitation, we evaluated the effect of the homologation of the alkyl side chain of the imidazolidin-2-one moiety of PAIB-SOs. Our studies evidence that PAIB-SOs bearing an n-pentyl side chain exhibit antiproliferative activity in the nanomolar-to-low-micromolar range and a high selectivity toward CYP1A1-positive breast cancer cells. Moreover, the most potent n-pentyl PAIB-SOs were significantly more stable toward rodent liver microsomes. In addition, PAIB-SOs 10 and 14 show significant antitumor activity and low toxicity in chorioallantoic membrane (CAM) assay. Our study confirms that homologation is a suitable approach to improve the rodent hepatic stability of PAIB-SOs.
中文翻译:

抗有丝分裂苯基 4-(2-Oxo-3-alkylimidazolidin-1-yl) 苯磺酸盐前药选择性靶向表达 CYP1A1 的乳腺癌的烷基侧链的同系化提高了它们在啮齿动物肝微粒体中的稳定性
苯基 4-(2-oxo-3-alkylimidazolidin-1-yl)benzenesulfonates (PAIB-SOs) 是一个新的抗有丝分裂前药家族,在表达 CYP1A1 的乳腺癌细胞中被生物激活。在这项研究中,我们报告了14 C 标记的原型 PAIB-SO [ 14 C]CEU-818 及其抗有丝分裂对应物 [ 14 C]CEU-602 分布在整个小鼠体内,并且它们在小鼠体内的半衰期较短. 为了规避这一限制,我们评估了 PAIB-SOs 的咪唑啉-2-one 部分的烷基侧链同系化的影响。我们的研究表明,PAIB-SO 具有n-戊基侧链在纳摩尔到低微摩尔范围内表现出抗增殖活性,并且对 CYP1A1 阳性乳腺癌细胞具有高选择性。此外,最有效的正戊基 PAIB-SO 对啮齿动物肝微粒体的稳定性明显更高。此外,PAIB-SO 10和14在绒毛尿囊膜 (CAM) 测定中显示出显着的抗肿瘤活性和低毒性。我们的研究证实,同系化是提高 PAIB-SO 啮齿动物肝脏稳定性的合适方法。
更新日期:2023-02-13
中文翻译:

抗有丝分裂苯基 4-(2-Oxo-3-alkylimidazolidin-1-yl) 苯磺酸盐前药选择性靶向表达 CYP1A1 的乳腺癌的烷基侧链的同系化提高了它们在啮齿动物肝微粒体中的稳定性
苯基 4-(2-oxo-3-alkylimidazolidin-1-yl)benzenesulfonates (PAIB-SOs) 是一个新的抗有丝分裂前药家族,在表达 CYP1A1 的乳腺癌细胞中被生物激活。在这项研究中,我们报告了14 C 标记的原型 PAIB-SO [ 14 C]CEU-818 及其抗有丝分裂对应物 [ 14 C]CEU-602 分布在整个小鼠体内,并且它们在小鼠体内的半衰期较短. 为了规避这一限制,我们评估了 PAIB-SOs 的咪唑啉-2-one 部分的烷基侧链同系化的影响。我们的研究表明,PAIB-SO 具有n-戊基侧链在纳摩尔到低微摩尔范围内表现出抗增殖活性,并且对 CYP1A1 阳性乳腺癌细胞具有高选择性。此外,最有效的正戊基 PAIB-SO 对啮齿动物肝微粒体的稳定性明显更高。此外,PAIB-SO 10和14在绒毛尿囊膜 (CAM) 测定中显示出显着的抗肿瘤活性和低毒性。我们的研究证实,同系化是提高 PAIB-SO 啮齿动物肝脏稳定性的合适方法。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号