当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chiral-boron-Lewis-acid-catalysed desymmetric ring expansion of 4-substituted cyclohexanones with α-diazomethylphosphonates
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2023-02-13 , DOI: 10.1039/d2qo01912d Xiaofeng Li 1 , Pingping He 1 , Peng Zhu 1 , Yinhong Tang 1 , Yungui Peng 1
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2023-02-13 , DOI: 10.1039/d2qo01912d Xiaofeng Li 1 , Pingping He 1 , Peng Zhu 1 , Yinhong Tang 1 , Yungui Peng 1
Affiliation
|
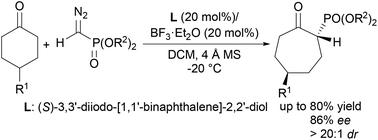
A method for desymmetrizing asymmetric ring expansion of 4-substituted cyclohexanones with α-diazomethylphosphonates catalyzed by a chiral boron Lewis acid has been developed. The method affords β-ketophosphonate compounds bearing a stereogenic center at the α-position in up to 80% yields, 86% ee, and >20 : 1 dr. The obtained products can be further derivatized, demonstrating the synthetic potential of this reaction.
中文翻译:

手性硼路易斯酸催化 4-取代环己酮与 α-重氮甲基膦酸酯的不对称扩环
开发了一种通过手性硼路易斯酸催化的 α-重氮甲基膦酸酯使 4-取代环己酮的不对称扩环去对称化的方法。该方法以高达 80% 的产率、86% ee 和 >20 : 1 dr 提供在 α 位带有立体异构中心的 β-酮膦酸酯化合物。所得产物可以进一步衍生化,证明了该反应的合成潜力。
更新日期:2023-02-13
中文翻译:

手性硼路易斯酸催化 4-取代环己酮与 α-重氮甲基膦酸酯的不对称扩环
开发了一种通过手性硼路易斯酸催化的 α-重氮甲基膦酸酯使 4-取代环己酮的不对称扩环去对称化的方法。该方法以高达 80% 的产率、86% ee 和 >20 : 1 dr 提供在 α 位带有立体异构中心的 β-酮膦酸酯化合物。所得产物可以进一步衍生化,证明了该反应的合成潜力。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号