当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thiol modifier effects of diphenyl diselenides: insight from experiment and DFT calculations
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2023-02-13 , DOI: 10.1039/d2nj05976b Pablo A. Nogara 1, 2 , Cláudia S. Oliveira 3, 4 , Andrea Madabeni 5 , Marco Bortoli 6 , João Batista T. Rocha 1 , Laura Orian 5
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2023-02-13 , DOI: 10.1039/d2nj05976b Pablo A. Nogara 1, 2 , Cláudia S. Oliveira 3, 4 , Andrea Madabeni 5 , Marco Bortoli 6 , João Batista T. Rocha 1 , Laura Orian 5
Affiliation
|
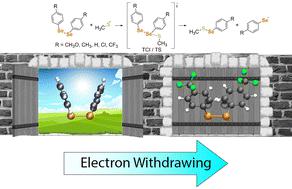
A combination of spectroscopic, chromatographic and computational approaches was employed to investigate the reaction of several diselenides of formula (R-PhSe)2 (R = CH3O, CH3, H, Cl, CF3) with a thiolate nucleophile, leading to the breaking of the selenium–selenium (Se–Se) bond. This process has fundamental importance in biological environments and provides a rationale to analyze the so-called thiol modifier effect of diselenides, which may be exploited in pharmacology and toxicology. Our data suggest that withdrawing substituents favor the reaction, effectively making the reaction energy more negative, but strong electron-withdrawing groups also prompt structural modification on the starting reactant, increasing the reaction barrier. Thus, the nature (electron rich or electron poor) of the diselenides can play an essential role in the reactivity and biological activity of these molecules.
中文翻译:

二苯基二硒化物的硫醇改性剂作用:来自实验和 DFT 计算的见解
采用光谱、色谱和计算方法的组合来研究式 (R-PhSe) 2 (R = CH 3 O, CH 3 , H, Cl, CF 3) 与硫醇亲核试剂,导致硒-硒 (Se-Se) 键断裂。该过程在生物环境中具有根本重要性,并为分析二硒化物所谓的硫醇改性剂效应提供了理论依据,可在药理学和毒理学中加以利用。我们的数据表明,撤回取代基有利于反应,有效地使反应能量更负,但强吸电子基团也促使起始反应物发生结构修饰,增加反应势垒。因此,二硒化物的性质(富电子或贫电子)可以在这些分子的反应性和生物活性中发挥重要作用。
更新日期:2023-02-13
中文翻译:

二苯基二硒化物的硫醇改性剂作用:来自实验和 DFT 计算的见解
采用光谱、色谱和计算方法的组合来研究式 (R-PhSe) 2 (R = CH 3 O, CH 3 , H, Cl, CF 3) 与硫醇亲核试剂,导致硒-硒 (Se-Se) 键断裂。该过程在生物环境中具有根本重要性,并为分析二硒化物所谓的硫醇改性剂效应提供了理论依据,可在药理学和毒理学中加以利用。我们的数据表明,撤回取代基有利于反应,有效地使反应能量更负,但强吸电子基团也促使起始反应物发生结构修饰,增加反应势垒。因此,二硒化物的性质(富电子或贫电子)可以在这些分子的反应性和生物活性中发挥重要作用。































 京公网安备 11010802027423号
京公网安备 11010802027423号