Nano Today ( IF 13.2 ) Pub Date : 2023-02-10 , DOI: 10.1016/j.nantod.2023.101769 Siyu Wang , Long Binh Vong , Zbynek Heger , Yue Zhou , Xiaoyang Liang , Vojtech Adam , Nan Li
|
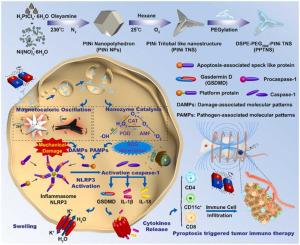
Pyroptosis, a unique form of programmed cell death, has been verified to be linked to inflammatory diseases and malignant tumors. Although great achievements have been made in pyroptosis research, several gaps, such as innate drug resistance and severe toxicity, hinder the development of pyroptosis inducers for biomedical applications. Thus, in this study, we designed a polyethylene glycol (PEG)ylated platinum-nickel (PtNi) bimetallic “trilobal”-shaped nanostructure (PPTNS) for effective pyroptosis-triggered tumor immunotherapy through a nanozyme catalytic and magnetocaloric oscillation dual strategy. Upon application of an alternating magnetic field, hyperthermia and mechanical oscillation of the specific sharp angles of PPTNS promoted damage-associated molecular pattern recognition, thereby activating the caspase-1-NLRP3-GSDMD pathway to increase cytokine recruitment. Furthermore, the high specific surface area and intrinsic nanozyme activities of PPTNS efficiently generated reactive oxygen species as the pathogen-associated molecular pattern, thus stimulating pattern recognition receptors to accelerate the oligomerization of the NOD-like receptor NLRP3. By this dual strategy, pyroptosis was triggered to perforate the cell membrane, resulting in cell rupture and cytokine release. After two weeks of treatment, the sizes of the breast tumors were significantly reduced without noticeable long-term toxicity in vivo. This strategy provides a magnetic-responsive platform for proptosis-triggered immunotherapy, which offers promising prospects for the highly efficient treatment of malignant tumors.
中文翻译:

基于 PtNi 纳米三叶形的纳米结构具有磁热振荡和对细胞焦亡触发的肿瘤免疫治疗的催化作用
细胞焦亡是一种独特的程序性细胞死亡形式,已被证实与炎症性疾病和恶性肿瘤有关。尽管细胞焦亡研究取得了巨大成就,但先天耐药性和严重毒性等一些差距阻碍了细胞焦亡诱导剂在生物医学应用中的发展。因此,在这项研究中,我们设计了聚乙二醇 (PEG) 化的铂镍 (PtNi) 双金属“三叶”形纳米结构 (PPTNS),通过纳米酶催化和磁热振荡双重策略进行有效的细胞焦亡触发的肿瘤免疫治疗。在应用交变磁场后,PPTNS 特定锐角的热疗和机械振荡促进了与损伤相关的分子模式识别,从而激活 caspase-1-NLRP3-GSDMD 通路以增加细胞因子募集。此外,PPTNS 的高比表面积和固有的纳米酶活性有效地产生活性氧作为病原体相关分子模式,从而刺激模式识别受体加速 NOD 样受体 NLRP3 的寡聚化。通过这种双重策略,细胞焦亡被触发穿孔细胞膜,导致细胞破裂和细胞因子释放。治疗两周后,乳腺肿瘤的大小明显缩小,而且没有明显的长期毒性 从而刺激模式识别受体加速 NOD 样受体 NLRP3 的寡聚化。通过这种双重策略,细胞焦亡被触发穿孔细胞膜,导致细胞破裂和细胞因子释放。治疗两周后,乳腺肿瘤的大小明显缩小,而且没有明显的长期毒性 从而刺激模式识别受体加速 NOD 样受体 NLRP3 的寡聚化。通过这种双重策略,细胞焦亡被触发穿孔细胞膜,导致细胞破裂和细胞因子释放。治疗两周后,乳腺肿瘤的大小明显缩小,而且没有明显的长期毒性体内。该策略为突眼触发的免疫治疗提供了一个磁响应平台,为恶性肿瘤的高效治疗提供了广阔的前景。































 京公网安备 11010802027423号
京公网安备 11010802027423号