Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2023-02-10 , DOI: 10.1016/j.apcatb.2023.122442 Xingdong Wang , Jinjie Fang , Xuerui Liu , Dong Wei , Yiquan Yin , Hailong Wei , Jinlin Zhang , Yufeng Zhang , Xuejiang Zhang , Wei Zhu , Zhongbin Zhuang
|
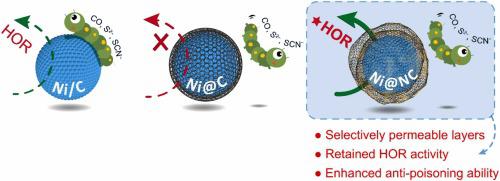
The use of platinum group metals (PGMs) as the catalysts and the requirement of high purity H2 are the two aspects resulting in the high cost of the polymer electrolyte membrane fuel cells. Herein, we report that nitrogen-doped carbon encapsulated nickel nanoparticles (Ni@NC) as stable hydrogen oxidation reaction catalysts, which allows the use of PGM-free anode catalysts durably and feeding crude hydrogen. The hydroxide exchange membrane fuel cell using Ni@NC as the anode catalyst illustrates steady performance for more than 200 h, while the Ni/C cell fails within 7 h. The current density loss is less than 10% by feeding H2 with 100 ppm of CO for 24 h, which is much better than those using Pt/C or Ni/C. The anti-poisoning feature is attributed to the block effect of the NC shell, while the defects allow the permeation of H2, thus catalyzing the hydrogen oxidation reaction.
中文翻译:

氮掺杂碳作为选择性渗透层增强氢氧化物交换膜燃料电池氢氧化反应催化剂的抗中毒能力
使用铂族金属(PGMs)作为催化剂和要求高纯度H 2是导致聚合物电解质膜燃料电池成本高的两个方面。在此,我们报道了氮掺杂碳包裹的镍纳米粒子 (Ni@NC) 作为稳定的氢氧化反应催化剂,这允许持久使用不含 PGM 的阳极催化剂并供给粗氢。使用 Ni@NC 作为阳极催化剂的氢氧化物交换膜燃料电池表现出稳定的性能超过 200 小时,而 Ni/C 电池在 7 小时内失效。通入H 2电流密度损失小于10%用 100 ppm 的 CO 处理 24 小时,这比使用 Pt/C 或 Ni/C 的要好得多。抗毒特性归因于NC壳的阻滞作用,而缺陷则允许H 2渗透,从而催化氢氧化反应。































 京公网安备 11010802027423号
京公网安备 11010802027423号