当前位置:
X-MOL 学术
›
Kidney Int. Rep.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Targeting the Alternative Complement Pathway With Iptacopan to Treat IgA Nephropathy: Design and Rationale of the APPLAUSE-IgAN Study
Kidney International Reports ( IF 5.7 ) Pub Date : 2023-02-09 , DOI: 10.1016/j.ekir.2023.01.041 Dana V Rizk 1 , Brad H Rovin 2 , Hong Zhang 3 , Naoki Kashihara 4 , Bart Maes 5 , Hernán Trimarchi 6 , Vlado Perkovic 7 , Matthias Meier 8 , Dmitrij Kollins 8 , Olympia Papachristofi 8 , Alan Charney 9 , Jonathan Barratt 10
Kidney International Reports ( IF 5.7 ) Pub Date : 2023-02-09 , DOI: 10.1016/j.ekir.2023.01.041 Dana V Rizk 1 , Brad H Rovin 2 , Hong Zhang 3 , Naoki Kashihara 4 , Bart Maes 5 , Hernán Trimarchi 6 , Vlado Perkovic 7 , Matthias Meier 8 , Dmitrij Kollins 8 , Olympia Papachristofi 8 , Alan Charney 9 , Jonathan Barratt 10
Affiliation
|
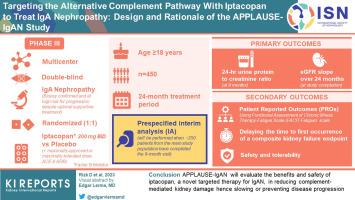
Targeting the alternative complement pathway (AP) is an attractive therapeutic strategy because of its role in immunoglobulin A nephropathy (IgAN) pathophysiology. Iptacopan (LNP023), a proximal complement inhibitor that specifically binds to factor B and inhibits the AP, reduced proteinuria and attenuated AP activation in a Phase 2 study of patients with IgAN, thereby supporting the rationale for its evaluation in a Phase 3 study. APPLAUSE-IgAN (NCT04578834) is a multicenter, randomized, double-blind, placebo-controlled, parallel-group, Phase 3 study enrolling approximately 450 adult patients (aged ≥18 years) with biopsy-confirmed primary IgAN at high risk of progression to kidney failure despite optimal supportive treatment. Eligible patients receiving stable and maximally tolerated doses of angiotensin-converting enzyme inhibitors (ACEis) or angiotensin receptor blockers (ARBs) will be randomized 1:1 to either iptacopan 200 mg or placebo twice daily for a 24-month treatment period. A prespecified interim analysis (IA) will be performed when approximately 250 patients from the main study population complete the 9-month visit. The primary objective is to demonstrate superiority of iptacopan over placebo in reducing 24-hour urine protein-to-creatinine ratio (UPCR) at the IA and demonstrate the superiority of iptacopan over placebo in slowing the rate of estimated glomerular filtration rate (eGFR) decline (total eGFR slope) estimated over 24 months at study completion. The effect of iptacopan on patient-reported outcomes, safety, and tolerability will be evaluated as secondary outcomes. APPLAUSE-IgAN will evaluate the benefits and safety of iptacopan, a novel targeted therapy for IgAN, in reducing complement-mediated kidney damage and thus slowing or preventing disease progression.
中文翻译:

使用 Iptacopan 靶向替代补体途径治疗 IgA 肾病:APPLAUSE-IgAN 研究的设计和原理
由于补体替代途径 (AP) 在免疫球蛋白 A 肾病 (IgAN) 病理生理学中的作用,靶向补体替代途径 (AP) 是一种颇具吸引力的治疗策略。 Iptacopan (LNP023) 是一种近端补体抑制剂,可特异性结合 B 因子并抑制 AP,在 IgAN 患者的 2 期研究中减少蛋白尿并减弱 AP 激活,从而支持其在 3 期研究中评估的基本原理。 APPLAUSE-IgAN (NCT04578834) 是一项多中心、随机、双盲、安慰剂对照、平行组 3 期研究,入组了大约 450 名经活检确诊为原发性 IgAN 且进展为高风险的成年患者(年龄≥18 岁)。尽管进行了最佳支持治疗,仍出现肾衰竭。接受稳定且最大耐受剂量的血管紧张素转换酶抑制剂 (ACEis) 或血管紧张素受体阻滞剂 (ARB) 的符合条件的患者将按 1:1 的比例随机分配至伊普塔考潘 200 mg 或安慰剂,每日两次,持续 24 个月的治疗期。当主要研究人群中约 250 名患者完成为期 9 个月的访视时,将进行预先指定的中期分析 (IA)。主要目标是证明 iptacopan 在降低 IA 24 小时尿蛋白肌酐比 (UPCR) 方面优于安慰剂,并证明 iptacopan 在减缓估计肾小球滤过率 (eGFR) 下降速度方面优于安慰剂(总 eGFR 斜率)在研究完成时估计超过 24 个月。 iptacopan 对患者报告的结果、安全性和耐受性的影响将作为次要结果进行评估。 APPLAUSE-IgAN 将评估 iptacopan(一种针对 IgAN 的新型靶向疗法)在减少补体介导的肾脏损伤,从而减缓或预防疾病进展方面的益处和安全性。
更新日期:2023-02-09
中文翻译:

使用 Iptacopan 靶向替代补体途径治疗 IgA 肾病:APPLAUSE-IgAN 研究的设计和原理
由于补体替代途径 (AP) 在免疫球蛋白 A 肾病 (IgAN) 病理生理学中的作用,靶向补体替代途径 (AP) 是一种颇具吸引力的治疗策略。 Iptacopan (LNP023) 是一种近端补体抑制剂,可特异性结合 B 因子并抑制 AP,在 IgAN 患者的 2 期研究中减少蛋白尿并减弱 AP 激活,从而支持其在 3 期研究中评估的基本原理。 APPLAUSE-IgAN (NCT04578834) 是一项多中心、随机、双盲、安慰剂对照、平行组 3 期研究,入组了大约 450 名经活检确诊为原发性 IgAN 且进展为高风险的成年患者(年龄≥18 岁)。尽管进行了最佳支持治疗,仍出现肾衰竭。接受稳定且最大耐受剂量的血管紧张素转换酶抑制剂 (ACEis) 或血管紧张素受体阻滞剂 (ARB) 的符合条件的患者将按 1:1 的比例随机分配至伊普塔考潘 200 mg 或安慰剂,每日两次,持续 24 个月的治疗期。当主要研究人群中约 250 名患者完成为期 9 个月的访视时,将进行预先指定的中期分析 (IA)。主要目标是证明 iptacopan 在降低 IA 24 小时尿蛋白肌酐比 (UPCR) 方面优于安慰剂,并证明 iptacopan 在减缓估计肾小球滤过率 (eGFR) 下降速度方面优于安慰剂(总 eGFR 斜率)在研究完成时估计超过 24 个月。 iptacopan 对患者报告的结果、安全性和耐受性的影响将作为次要结果进行评估。 APPLAUSE-IgAN 将评估 iptacopan(一种针对 IgAN 的新型靶向疗法)在减少补体介导的肾脏损伤,从而减缓或预防疾病进展方面的益处和安全性。































 京公网安备 11010802027423号
京公网安备 11010802027423号