Chinese Chemical Letters ( IF 9.4 ) Pub Date : 2023-02-11 , DOI: 10.1016/j.cclet.2023.108206 Chunyang Zhang , Yuelan Li , Zhaojun Chu , Shuangzhi Yuan , Yanan Qiao , Jiaozhen Zhang , Lin Li , Yueqing Zhang , Ruifeng Tian , Yajie Tang , Hongxiang Lou
|
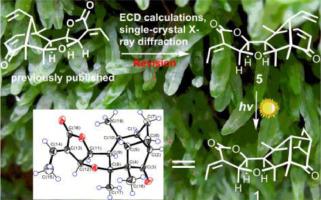
Two distinctive rearranged 19-nor-7,8-seco-labdane diterpenoids (1 and 2) with a novel tetracyclo[5.2.1.02,5.04,10]decane skeleton, a derivative of the open tetrahydrofuran ring (7), three dimeric compounds (8−10), and four revised homologs (3−6) were obtained from Chinese liverwort Pallavicinia ambigua. Their structures were identified via combined analysis of their spectroscopic data, single-crystal X-ray diffraction patterns, and ECD calculations. The light-driven conversion of compound 5 to compounds 1−4 demonstrated that photochemically induced postmodification involved in biosynthesis is an important way to diversify natural structures. A preliminary cytotoxicity assay revealed that compound 5 showed significant inhibition in the human prostate cancer (PC-3) cell line via an apoptotic pathway.































 京公网安备 11010802027423号
京公网安备 11010802027423号