Diamond and Related Materials ( IF 4.3 ) Pub Date : 2023-02-10 , DOI: 10.1016/j.diamond.2023.109773
Mustafa M. Kadhim , Ammar Abdulkadhim , Safa K. Hachim , Sallal A.H. Abdullaha , Taleeb Zedan Taban , Ahmed Mahdi Rheima
|
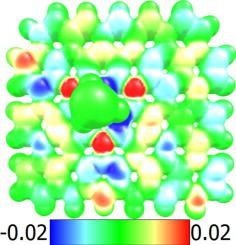
Due to high affinity and extensive surface area between arsenic and nitrogen in C3N, the two-dimensional C3N monolayer has considerable the potential to effectively adjust the release of gaseous As2O3. By performing density functional theory (DFT) calculations, the adsorption of As2O3 on the surface of C3N monolayer was investigated. The results revealed that site C4N2 on the surface of the C3N monolayer has greater ability than site C6 to strongly adsorb As2O3. Also, the recovery time of the C3N monolayer for As2O3 were about 25.64 s and 5.23 μs at 298 and 400 K, respectively. The results related to the charge transfer (CT), adsorption stability, electronic and geometric structure confirmed that the C3N monolayer was an appropriate choice for adsorption of As2O3. Overall, the C3N monolayer can be considered as one of the most suitable adsorbents that can be used to effectively control gaseous As2O3.
中文翻译:

使用 C3N 单层吸附剂去除气态 As2O3:DFT 研究
由于C 3 N中砷和氮之间的高亲和力和大表面积,二维C 3 N单层具有相当大的潜力来有效调节气态As 2 O 3的释放。通过密度泛函理论(DFT)计算,研究了As 2 O 3在C 3 N单层表面的吸附。结果表明,C 3 N单分子层表面的C 4 N 2 位比C 6位具有更强的吸附As 2 O 3的能力。此外,C的恢复时间As 2 O 3的3 N 单层在 298 和 400 K 时分别约为 25.64 s 和 5.23 μs。与电荷转移 (CT)、吸附稳定性、电子和几何结构相关的结果证实,C 3 N 单分子层是吸附 As 2 O 3的合适选择。总的来说,C 3 N单分子层可以被认为是最适合用于有效控制气态As 2 O 3的吸附剂之一。

































 京公网安备 11010802027423号
京公网安备 11010802027423号