Insect Conservation and Diversity ( IF 3.2 ) Pub Date : 2023-02-09 , DOI: 10.1111/icad.12630 James Cranston 1 , Nick J. B. Isaac 2 , Regan Early 1
|
INTRODUCTION
Novel species interactions are occurring around the world as anthropogenic environmental change causes species distributions to shift with increasing frequency (Bonebrake et al., 2018; Gurney, 2015; Mason et al., 2015). These interactions may lead to a range of both positive and negative effects, often depending on the local environmental and social context (Pecl et al., 2017; Pettorelli et al., 2019). Despite their potential to alter recipient ecosystems, species that are losing historic range will be at increased risk of extinction if they cannot establish in new areas (Araújo et al., 2011; Thomas et al., 2004). Therefore, we may sometimes see a conservation dilemma between protecting the new arrivals and protecting historically resident species.
Range-shifters, that is, species immigrating to novel communities without human assistance, are often expected to have minimal effects on recipient ecosystems (Urban, 2020; Wallingford et al., 2020; Wilson et al., 2016). Nevertheless, some studies have found negative impacts such as: out-competing residents (Fitt & Lancaster, 2017; Yackulic et al., 2019), disease spill over (Dobson, 2009), direct predation, and hybridisation (Sánchez-Guillén et al., 2013; Steeves et al., 2010). On the other hand, species that are losing historic range will be at increased risk of extinction if they cannot establish in new areas (Araújo et al., 2011; Thomas et al., 2004). Understanding local factors associated with range-shifter establishment, including the resident species with which they are co-occurring, is therefore important for conservation planning for both resident and range-shifting species. It therefore seems prudent to develop tools to assess how range-shifters integrate into resident biota, and whether undesirable effects are occurring.
Horizon scanning (Roy et al., 2014), risk assessment (Hawkins et al., 2015) and proactive monitoring (Kennedy et al., 2018) are often used to identify threats from introduced invasive species. However, such efforts, as well as legal or management frameworks for alien species, focus on species that have been introduced by humans—not range-shifting species moving under their own powers of dispersal (Trouwborst et al., 2015). The large number of range-shifters (Lenoir et al., 2020) and concomitant stress from changes to the physical environment suggests we should not overlook this phenomenon. Being able to monitor and quantify the potential for novel interactions between range-shifters and residents is important for two reasons. First, in the short term, quantification allows limited conservation resources to be spent on the species of greatest impact or need (Carrasco et al., 2010; Kumschick et al., 2012). Second, in the longer term, the resultant knowledge base could be used to compare the impacts of range-shifting species at regional and global levels relative to other risks to biodiversity (Turbelin et al., 2017). However, detecting novel interactions is challenging. There is little systematic data on range-shifters' distributions in recently occupied parts of their ranges and even less on their abundance or interactions with residents. Most available data are opportunistically collected rather than using standard protocols (Amorim et al., 2014). Unfortunately, opportunistic data can suffer from several biases, which can lead to flawed inferences over species trends (Isaac & Pocock, 2015). Furthermore, it can be challenging to separate the effects of the arriving species from other environmental changes. If environmental change increases both the probability a new species will establish and the probability a resident species will go locally extinct, this could lead to a correlation between new species arrival and resident species decline (Parmesan et al., 2011). Climate change is a quintessential example. It allows new thermophilic species to establish but by exceeding current residents' thermal limits causes their probability of persistence to decrease (Pinkert et al., 2022). These indirect associations, as well as sampling biases, mean that we should be cautious when inferring biotic interactions from correlations between resident and range-shifting species using opportunistic data (Blanchet et al., 2020; Dormann et al., 2018). Nonetheless, one advantage of modelling over (most) experimental studies, in that it can integrate data from a much wider geographic area and time period. Moreover, models can be more easily replicated and adapted, for example, different environmental covariates or different model formulations can be statistically compared, than field or laboratory experiments can be rerun. Therefore, modelling the occupancy of multiple species using opportunistic data can act as useful starting points for generating hypotheses and general patterns so long as consideration is paid to the risk of assigning causality.
Dynamic multispecies occupancy models (DMSOs) have been used successfully to link environmental change to biodiversity impacts using occurrence records (Woodcock et al., 2016). Including environmental covariates can help to rule out possible confounding factors such as climate or habitat. In addition, including ecological processes such as dispersal in sub-models can also help to build more biologically realistic models. Our understanding of range-shifters effects can also be updated as more data become available to estimate parameters, allowing near real-time inputs into conservation strategy (Mancini et al., 2019).
Here, we apply DMSOs to examine a range-shifting species—the small red-eyed or small redeye damselfly (Erythromma viridulum). The species was first detected in the United Kingdom in 1999 and has continued to establish over the last two decades. Originally a Mediterranean species, the damselfly has spread gradually northwards, arriving in the United Kingdom via multiple irruptive waves from 1999 and becoming established by 2002, at times appearing abundant at certain sites (Watts et al., 2010). The damselfly is at the current poleward edge of its distribution in the United Kingdom (GBIF Secretariat, 2019) and thus breeds less frequently there, either annually or biennially, than in the warmer core of its range. It favours static pools or slow-flowing rivers for egg-laying as they have requisite macrophytes, particularly Hornwort and Water Milfoil. It flies principally between May and September (Brooks & Cham, 2014).
E. viridulum is a representative and timely study species for investigating measures of impact since several new Odonata are expected to establish in upcoming years (Parr, 2010). There are reasons to think range-shifting Odonata both may and may not have effects on UK resident Odonata. Odonata are known for their strong inter-specific interactions in both their adult and larval stages, including predation, which constitutes a biologically plausible mechanism for impact (Cerini et al., 2019; Wissinger & McGrady, 1993). There are numerous examples of range-shifting Odonata with competitive advantages over resident species (Pinkert et al., 2022 and references therein; Siepielski et al., 2022). Interactions in the larval stage are hard to observe directly and therefore inferring through modelling could be particularly useful. In addition, the UK Odonata is relatively species-poor compared to similar latitudes in mainland Europe (Kalkman et al., 2018). This is concerning as low biodiversity has been shown to decrease resistance to invasion (Kennedy et al., 2002). Impacts may also be amplified since several UK Odonata species are already locally threatened (Daguet et al., 2008). Odonata are range-shifting rapidly within the United Kingdom and Europe, so are of particular interest (Pinkert et al., 2022). Within the Odonata, E. viridulum is one of two species (both damselflies) to have arrived in the United Kingdom within recent decades and is the most numerous (Parr, 2010; Watts et al., 2010). It displays aggressive flight behaviour towards six resident Odonata and has high dietary overlap with at least one other damselfly (Coenagrion puella, Cox, 2013). E. viridulum is one of the most rapidly range-shifting species in Europe, it forms large populations where it establishes (Trippier et al., 2014), can become one of the most abundant species in the odonate community (Ketalaar, 2002). E. Viridulum seems to be showing some form of adaptation to local conditions (Hassall et al., 2014), which can alter the balance of inter-specific interactions (Lancaster et al., 2017; Therry et al., 2014). These traits, often found in other range-shifting odonata (Pinkert et al., 2022), mirror those found in many invasive species (Estrada et al., 2016; Wallingford et al., 2020). E. viridulum has a congener in the United Kingdom (Erythromma najas) with whom it overlaps in habitat preference and flight season (Powney et al., 2014). This could result in a negative impact, or the presence of E. najas could stall the establishment of E. viridulum.
On the other hand, Odonata have fairly generalist diets, meaning that competition for food and intraguild predation between E. viridulum and most resident species may be low unless external conditions led to severe food shortages. E. viridulum's short flight periods might also minimise the effects of territoriality. In addition, low Odonata species richness in the United Kingdom may mean that there are vacant niches to exploit (Gauzere et al., 2020), reducing other forms of resource competition. Therefore, some resident Odonata, particularly the larger dragonflies, may be unlikely to suffer negative effects from E. viridulum, than others, for example, the damselflies. Because the larger dragonflies are well recorded through the same methods as other Odonata and found in the same habitat, they potentially provide a good control. If negative associations between E. viridulum and many species of dragonflies were found, this could indicate a third factor is causing joint declines, and confounding detection of biotic interactions.
Data on E. viridulum are some of the best available to test our approach given that the United Kingdom has some of the best Odonata recording globally. Within our study area, a team of 51 volunteer County Dragonfly Recorders (CDRs) solicit, collect, collate and verify records contributed by recorders over a fairly small geographic area, manually determining whether a record is accepted (Taylor et al., 2021). CDRs know local sites very well, and given that the arrival of E. viridulum has generated substantial interest, CDRs are likely to verify new sightings in their vice county rigorously. The rigour with which records of many potentially interacting species are verified means that DMSO techniques may be readily and accurately applied. We note that there is potential for misidentification between E. viridulum and E. najas. The added noise in the data is likely to make it harder for models to detect species associations, rather than detect spurious associations.
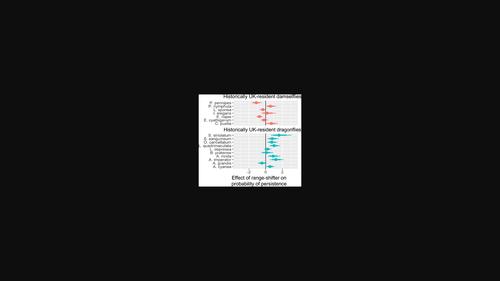
In this study, we investigated whether we could detect and quantify associations between the occurrences of the range-shifting E. viridulum and the UK's resident Odonata. Specifically, we asked whether there was a net change in the persistence probability of dragonflies (Anisoptera) and damselflies (Zygoptera) at sites where E. viridulum had arrived. We also examined changes in the persistence of individual species. Our study incorporated data from 2609 1 km2 sites, 49,788 site visits from 2000 to 2015, 10 historically resident dragonfly species and 7 historically resident damselfly species, compensating for differential recorder effort through a detectability sub-model. We controlled for the potentially confounding effects of climate and recorder effort.
中文翻译:

变化范围的豆娘 (Erythromma viridulum) 与英国居民 Odonata 之间的关联表明栖息地共享比对抗更重要
介绍
新的物种相互作用正在世界各地发生,因为人为环境变化导致物种分布以越来越高的频率发生变化(Bonebrake 等人, 2018 年;Gurney, 2015 年;Mason 等人, 2015 年)。这些相互作用可能会导致一系列积极和消极的影响,通常取决于当地的环境和社会背景(Pecl 等人, 2017 年;Pettorelli 等人, 2019 年)。尽管它们有可能改变受体生态系统,但正在失去历史分布范围的物种如果不能在新地区定居,将面临更大的灭绝风险(Araújo 等人,2011 年;Thomas 等人, 2004年 )). 因此,我们有时可能会看到保护新来者和保护历史居民物种之间的保护困境。
范围转移者,即在没有人类帮助的情况下迁移到新社区的物种,通常预计对接受生态系统的影响最小(Urban, 2020 年;Wallingford 等人, 2020 年;Wilson 等人, 2016 年)。然而,一些研究发现了负面影响,例如:竞争激烈的居民(Fitt & Lancaster, 2017 年;Yackulic 等人, 2019 年)、疾病溢出(Dobson, 2009 年)、直接捕食和杂交(Sánchez-Guillén 等人) ., 2013 年;Steeves 等人, 2010 年)。另一方面,正在失去历史分布范围的物种如果不能在新地区定居,将面临更大的灭绝风险(Araújo 等人, 2011年;托马斯等人, 2004 年)。因此,了解与范围转移者建立相关的当地因素,包括与它们共同出现的常驻物种,对于常驻和范围转移物种的保护规划都很重要。因此,开发工具来评估变距因子如何融入常驻生物群,以及是否正在发生不良影响似乎是明智的。
地平线扫描(Roy et al., 2014)、风险评估(Hawkins et al., 2015)和主动监测(Kennedy et al., 2018)通常用于识别外来入侵物种的威胁。然而,此类努力以及针对外来物种的法律或管理框架都侧重于人类引入的物种,而不是在自身传播能力下移动的范围转移物种(Trouwborst 等人,2015 年 )。大量的移距器(Lenoir 等人, 2020) 以及物理环境变化带来的压力表明我们不应忽视这一现象。出于两个原因,能够监测和量化变距者与居民之间新型互动的潜力很重要。首先,在短期内,量化允许将有限的保护资源用于影响最大或需求最大的物种(Carrasco 等人, 2010 年;Kumschick 等人, 2012 年)。其次,从长远来看,由此产生的知识库可用于比较范围转移物种在区域和全球层面相对于其他生物多样性风险的影响(Turbelin 等人,2017 年 )). 然而,检测新的相互作用具有挑战性。几乎没有关于范围转移者在其范围内最近被占领部分的分布的系统数据,关于它们的数量或与居民互动的系统数据就更少了。大多数可用数据是随机收集的,而不是使用标准协议(Amorim 等人, 2014 年)。不幸的是,机会主义数据可能存在多种偏差,这可能导致对物种趋势的推断有缺陷(Isaac & Pocock, 2015 年)). 此外,将到达物种的影响与其他环境变化分开可能具有挑战性。如果环境变化增加了新物种建立的可能性和常驻物种在当地灭绝的可能性,这可能会导致新物种到来与常驻物种减少之间的相关性(Parmesan 等人,2011 年 )。气候变化就是一个典型的例子。它允许新的嗜热物种建立,但超过当前居民的热极限会导致其持久存在的可能性降低(Pinkert 等人, 2022 年)). 这些间接关联以及抽样偏差意味着我们在使用机会主义数据从常驻物种和范围转移物种之间的相关性推断生物相互作用时应该谨慎(Blanchet 等人,2020 年;Dormann 等人, 2018年 )). 尽管如此,建模优于(大多数)实验研究的一个优势在于它可以整合来自更广泛地理区域和时间段的数据。此外,模型可以更容易地复制和调整,例如,可以统计比较不同的环境协变量或不同的模型公式,而不是可以重新运行现场或实验室实验。因此,只要考虑分配因果关系的风险,使用机会数据对多个物种的占有率进行建模可以作为生成假设和一般模式的有用起点。
动态多物种占用模型 (DMSO) 已成功用于使用事件记录将环境变化与生物多样性影响联系起来(Woodcock 等人, 2016 年)。包括环境协变量有助于排除可能的混杂因素,例如气候或栖息地。此外,在子模型中包含扩散等生态过程也有助于构建更符合生物学现实的模型。随着越来越多的数据可用于估计参数,我们对变距效应的理解也可以得到更新,从而允许对保护策略进行近乎实时的输入(Mancini 等人,2019 年 )。
在这里,我们应用 DMSO 来检测范围变化的物种——小红眼或小红眼豆娘 ( Erythromma viridulum )。该物种于 1999 年在英国首次被发现,并在过去二十年中继续建立。豆娘最初是地中海物种,逐渐向北传播,从 1999 年起通过多次冲击波抵达英国,并于 2002 年定居,有时在某些地点出现数量众多(Watts 等人,2010 年 )。豆娘目前处于其在英国分布的极地边缘(GBIF 秘书处, 2019),因此在那里的繁殖频率较低,无论是每年还是每两年一次,都低于其分布范围较温暖的核心区域。它喜欢在静止的水池或缓慢流动的河流中产卵,因为它们有必需的大型植物,尤其是金鱼藻和水螅。它主要在 5 月和 9 月之间飞行(Brooks & Cham, 2014 年)。
E. viridulum是一种具有代表性且及时的研究物种,用于调查影响措施,因为预计在未来几年将建立几种新的蜻蜓目(Parr, 2010)。有理由认为范围转移的蜻蜓目可能会对英国居民蜻蜓目产生影响,也可能不会产生影响。众所周知,蜻蜓目在成虫和幼虫阶段都具有很强的种间相互作用,包括捕食,这构成了生物学上合理的影响机制(Cerini 等人,2019 年;Wissinger 和 McGrady, 1993年 )。有许多与常驻物种相比具有竞争优势的变化范围的蜻蜓目示例(Pinkert 等人, 2022 年及其中的参考资料;Siepielski 等人, 2022 年)). 幼虫阶段的相互作用很难直接观察,因此通过建模进行推断可能特别有用。此外,与欧洲大陆类似纬度地区相比,英国蜻蜓目物种相对较少(Kalkman 等人, 2018 年)。这是令人担忧的,因为低生物多样性已被证明会降低对入侵的抵抗力(Kennedy 等人, 2002 年)。由于英国的几种蜻蜓目物种已经在当地受到威胁,影响也可能会扩大(Daguet 等人, 2008 年)。Odonata 在英国和欧洲范围内迅速转移,因此特别令人感兴趣(Pinkert 等人, 2022 年)。在 Odonata 内,E. viridulum是近几十年来抵达英国的两个物种之一(均为豆娘),而且数量最多(Parr, 2010 年;Watts 等人, 2010 年)。它对六只常驻蜻蜓表现出攻击性的飞行行为,并且与至少一只其他豆娘有高度的饮食重叠 ( Coenagrion puella , Cox, 2013 )。E. viridulum是欧洲变化范围最快的物种之一,它在它建立的地方形成大量种群(Trippier 等人, 2014 年),可以成为 odonate 群落中最丰富的物种之一(Ketalaar, 2002 年)。E. 绿藻似乎表现出某种形式的适应当地条件(Hassall 等人, 2014 年),这可以改变种间相互作用的平衡(Lancaster 等人, 2017 年;Therry 等人, 2014 年)。这些特征通常在其他范围转移的 odonata 中发现(Pinkert 等人, 2022 年),反映了在许多入侵物种中发现的特征(Estrada 等人, 2016 年;Wallingford 等人, 2020 年)。E. viridulum在英国有一个同系物(Erythromma najas),它在栖息地偏好和飞行季节方面与其重叠(Powney 等人, 2014 年)。这可能会导致负面影响,或者E. najas的存在可以阻止E. viridulum的建立。
另一方面,蜻蜓目具有相当多才多艺的饮食,这意味着除非外部条件导致严重的食物短缺,否则E. viridulum和大多数常驻物种之间的食物竞争和内部捕食可能很低。E. viridulum 的短飞行期也可能将地域性的影响降到最低。此外,英国的蜻蜓目物种丰富度低可能意味着有空缺的生态位可供开发(Gauzere 等人, 2020 年),从而减少其他形式的资源竞争。因此,一些常驻蜻蜓,尤其是体型较大的蜻蜓,可能不太可能受到绿色桉树的负面影响,比其他人,例如,豆娘。因为较大的蜻蜓通过与其他蜻蜓目相同的方法被很好地记录下来并且在相同的栖息地发现,它们可能提供良好的控制。如果发现E. viridulum和许多蜻蜓物种之间存在负相关,这可能表明第三个因素导致关节衰退,并混淆了生物相互作用的检测。
考虑到英国在全球范围内拥有一些最好的蜻蜓目记录,因此关于E. viridulum 的数据是测试我们方法的最佳可用数据。在我们的研究区域内,一个由 51 名志愿县蜻蜓记录员 (CDR) 组成的团队在相当小的地理区域内征集、收集、整理和验证记录员提供的记录,并手动确定记录是否被接受(Taylor 等人,2021 年 )。CDR 非常了解本地站点,并且鉴于E. viridulum的到来引起了极大的兴趣,CDR 可能会严格核实其副县的新目击事件。验证许多潜在相互作用物种的记录的严格性意味着可以轻松准确地应用 DMSO 技术。我们注意到在E. viridulum和E. najas之间存在错误识别的可能性。数据中增加的噪音可能会使模型更难检测物种关联,而不是检测虚假关联。
在这项研究中,我们调查了我们是否可以检测和量化范围变化的E. viridulum与英国居民 Odonata 之间的关联。具体来说,我们询问在E. viridulum到达的地点,蜻蜓 (Anisoptera) 和豆娘 (Zygoptera) 的持久性概率是否有净变化。我们还检查了单个物种持久性的变化。我们的研究整合了来自 2609 个 1 km 2站点的数据、2000 年至 2015 年的 49,788 次站点访问、10 种历史上居住的蜻蜓物种和 7 种历史上居住的豆娘物种,通过可检测性子模型补偿差异记录器的工作。我们控制了气候和记录器工作的潜在混杂影响。































 京公网安备 11010802027423号
京公网安备 11010802027423号