Fuel ( IF 6.7 ) Pub Date : 2023-02-10 , DOI: 10.1016/j.fuel.2023.127664
Zhaoying Li , Qirong Yang , Li Tao , Xinru Ma , Jie Zhou , Tao Ye , Jinhu Wu , Ronghua Wu , Haoxi Ben
|
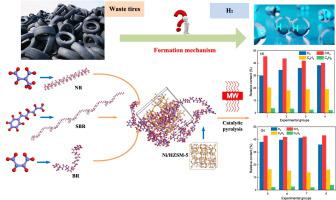
The mechanism for the formation of gaseous products, especially hydrogen, from the catalytic pyrolysis of waste tires by using Ni-based catalysts has been studied. The catalytic pyrolysis of the three essential components of waste tires such as natural rubber (NR), styrene-butadiene rubber (SBR), and cis-polybutadiene rubber (BR) was simulated and the pyrolysis fracture of the molecular chain was investigated. This work aims to provide a deeper understanding of the reaction thermodynamics and kinetics for the formation mechanisms of gaseous products, primarily hydrogen. Microwave-assisted catalytic pyrolysis of the waste tires over Ni-based catalysts was carried out and used to assent to the simulation results. The study revealed that with the incorporation of 10 wt% Ni/ZSM-5 catalyst, the relative content of H2 from the catalytic pyrolysis of waste tires can be significantly improved by about 41.3 % in comparison to the same measurement done in the absence of the catalyst. Kinetic and thermodynamic simulations for the catalytic pyrolysis of waste tires were also performed and the corresponding energy barriers and Gibbs free energy changes were calculated. Accordingly, the optimal reaction path for the hydrogen production was determined by comparing and analyzing the values of those parameters. It was confirmed that the addition of Ni, ZSM-5, and Ni/ZSM-5 catalysts promote the hydrogen production reaction pathways. Among those catalysts, the simulation result verified that Ni/ZSM-5 brings a pronounced hydrogen production capacity, which was consistent with the experimental findings.
中文翻译:

废轮胎催化热解制氢的形成机理:ReaxFF分子动力学与实验研究
已经研究了使用镍基催化剂从废轮胎催化热解中形成气态产物,尤其是氢气的机理。天然橡胶(NR)、丁苯橡胶(SBR)、顺式等废旧轮胎三大主要成分的催化热解- 模拟了聚丁二烯橡胶 (BR) 并研究了分子链的热解断裂。这项工作旨在更深入地了解气态产物(主要是氢)的形成机制的反应热力学和动力学。在镍基催化剂上进行了废轮胎的微波辅助催化热解,并用于验证模拟结果。研究表明,加入 10 wt% Ni/ZSM-5 催化剂后,H 2的相对含量与在没有催化剂的情况下进行的相同测量相比,废轮胎的催化热解产生的碳含量可以显着提高约 41.3%。还对废轮胎的催化热解进行了动力学和热力学模拟,并计算了相应的能垒和吉布斯自由能变化。因此,通过比较和分析这些参数的值,确定了制氢的最佳反应路径。已证实添加 Ni、ZSM-5 和 Ni/ZSM-5 催化剂促进了制氢反应途径。在这些催化剂中,模拟结果证实Ni/ZSM-5具有显着的产氢能力,这与实验结果一致。

































 京公网安备 11010802027423号
京公网安备 11010802027423号