Phytomedicine ( IF 6.7 ) Pub Date : 2023-02-08 , DOI: 10.1016/j.phymed.2023.154712 Li Fan 1 , Ying Peng 1 , Xiaobo Li 1
|
Background
Hydroxytyrosol (HT), as the main compound in olive leaves with its potential ability to cross blood-brain barrier (BBB), has exhibited the advantaged antidepressant effect. However, no information is available regarding the brain regional uptake of HT, as well the underlying antidepressant mechanism remains unclear.
Purpose
To comprehensively reveal the brain uptake of HT and its specific mechanism on the accompanying antidepressant activity.
Study design and methods
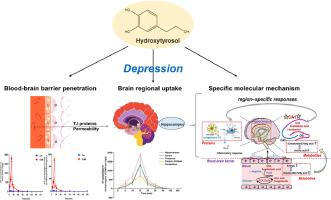
The BBB penetration and brain regional distribution of HT in the normal and chronic unpredictable mild stress (CUMS)–induced depressive mice in consideration with the BBB integrality were analyzed. Then, the hippocampal region–specific responses of biomolecules and concurrent alterations in the therapeutic effect of HT on depression were explored using untargeted metabolomics, spatial–resolved metabolomics and tissue proteomics, which were confirmed by LPS–induced BV–2 microglia and CUMS mice.
Results
BBB permeability analysis in normal and CUMS mice confirmed that increased BBB permeability of CUMS mice was induced by the deficiency of tight junction-related proteins. Consistently, according to the established LC–MS/MS method, it was found that HT could not be largely detected in the cerebrospinal fluids and brains of normal mice after oral administration, while it could excessively penetrate the BBB (200–fold higher), and mostly distributed in the hippocampus of CUMS mice. Meanwhile, multi–omics analysis combined with targeted analysis discovered that HT could mainly improve fatty acid biosynthesis and metabolism in the hippocampus with region–specific responses and accompanying inhibition of C3–CD11b pathway in CUMS mice. Besides, in vitro experiments further confirmed the anti–complement ability of HT, which could inhibit C3–CD11b pathway for alleviating the LPS–induced BV–2 microglia activation.
Conclusion
HT can excessively penetrate the BBB and be mostly distributed in the hippocampus of depressive mice, which contribute to improve fatty acid biosynthesis and metabolism in the hippocampus with region–specific responses and accompanying inhibition of C3–CD11b pathway for microglia activation. These findings give the clearer understanding of brain regional pharmacokinetics of HT and its accompanying molecular mechanism against depression.
中文翻译:

多组学方法评估羟基酪醇的脑区域药代动力学及其抗抑郁的分子机制
背景
羟基酪醇 (HT) 作为橄榄叶中的主要化合物,具有穿过血脑屏障 (BBB) 的潜在能力,表现出优越的抗抑郁作用。然而,没有关于 HT 的大脑区域摄取的信息,以及潜在的抗抑郁机制仍不清楚。
目的
全面揭示 HT 的大脑摄取及其对伴随的抗抑郁活性的具体机制。
研究设计和方法
考虑到 BBB 完整性,分析了 HT 在正常和慢性不可预测轻度应激 (CUMS) 诱导的抑郁小鼠中的 BBB 渗透和脑区域分布。然后,使用非靶向代谢组学、空间分辨代谢组学和组织蛋白质组学探索了生物分子的海马区域特异性反应和 HT 对抑郁症治疗效果的同时改变,这些被 LPS 诱导的 BV-2 小胶质细胞和 CUMS 小鼠证实。
结果
正常和 CUMS 小鼠的 BBB 通透性分析证实,CUMS 小鼠的 BBB 通透性增加是由紧密连接相关蛋白的缺乏引起的。一致地,根据已建立的 LC-MS/MS 方法,发现口服给药后在正常小鼠的脑脊液和大脑中无法大量检测到 HT,但它可以过度穿透 BBB(高 200 倍),且主要分布于 CUMS 小鼠的海马区。同时,多组学分析结合靶向分析发现,HT主要改善CUMS小鼠海马区脂肪酸的生物合成和代谢,具有区域特异性反应,并伴随抑制C3-CD11b通路。此外,体外实验进一步证实了 HT 的抗补体能力,可抑制 C3-CD11b 通路,减轻 LPS 诱导的 BV-2 小胶质细胞激活。
结论
HT 可以过度穿透 BBB 并且主要分布在抑郁小鼠的海马体中,这有助于改善海马体中的脂肪酸生物合成和代谢,具有区域特异性反应并伴随抑制 C3-CD11b 通路以激活小胶质细胞。这些发现使人们更清楚地了解 HT 的脑区域药代动力学及其伴随的抗抑郁分子机制。

































 京公网安备 11010802027423号
京公网安备 11010802027423号