Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2023-02-10 , DOI: 10.1016/j.jmb.2023.168009 Frank Heinrich 1 , Catherine E Thomas 2 , John J Alvarado 2 , Rebecca Eells 3 , Alyssa Thomas 3 , Mathieu Doucet 4 , Kindra N Whitlatch 2 , Manish Aryal 2 , Mathias Lösche 1 , Thomas E Smithgall 2
|
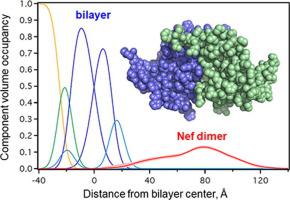
The HIV-1 Nef protein plays a critical role in viral infectivity, high-titer replication in vivo, and immune escape of HIV-infected cells. Nef lacks intrinsic biochemical activity, functioning instead through interactions with diverse host cell signaling proteins and intracellular trafficking pathways. Previous studies have established an essential role for Nef homodimer formation at the plasma membrane for most if not all its functions. Here we combined neutron reflectometry of full-length myristoylated Nef bound to model lipid bilayers with molecular simulations based on previous X-ray crystal structures of Nef homodimers. This integrated approach provides direct evidence that Nef associates with the membrane as a homodimer with its structured core region displaced from the membrane for partner protein engagement. Parallel studies of a dimerization-defective mutant, Nef-L112D, demonstrate that the helical dimerization interface present in previous crystal structures stabilizes the membrane-bound dimer. X-ray crystallography of the Nef-L112D mutant in complex with the SH3 domain of the Nef-associated host cell kinase Hck revealed a monomeric 1:1 complex instead of the 2:2 dimer complex formed with wild-type Nef. Importantly, the crystal structure of the Nef-L112D core and SH3 interface are virtually identical to the wild-type complex, indicating that this mutation does not affect the overall Nef fold. These findings support the intrinsic capacity of Nef to homodimerize at lipid bilayers using structural features present in X-ray crystal structures of dimeric complexes.
中文翻译:

中子反射法和分子模拟证明 HIV-1 Nef 同二聚体在模型脂质双层上形成
HIV-1 Nef蛋白在病毒感染性、体内高滴度复制以及HIV感染细胞的免疫逃逸中发挥着关键作用。 Nef 缺乏内在的生化活性,而是通过与不同宿主细胞信号蛋白和细胞内运输途径的相互作用发挥作用。先前的研究已经确定了质膜上 Nef 同型二聚体的形成对于其大部分功能(如果不是全部)的重要作用。在这里,我们将与模型脂质双层结合的全长肉豆蔻酰化 Nef 的中子反射测量与基于以前的 Nef 同二聚体的 X 射线晶体结构的分子模拟相结合。这种综合方法提供了直接证据,证明 Nef 作为同二聚体与膜结合,其结构核心区域从膜上移出以与伙伴蛋白结合。对二聚化缺陷突变体 Nef-L112D 的平行研究表明,先前晶体结构中存在的螺旋二聚化界面稳定了膜结合二聚体。与 Nef 相关宿主细胞激酶 Hck 的 SH3 结构域复合的 Nef-L112D 突变体的 X 射线晶体学显示,这是一个单体 1:1 复合物,而不是与野生型 Nef 形成的 2:2 二聚体复合物。重要的是,Nef-L112D 核心和 SH3 界面的晶体结构实际上与野生型复合物相同,表明这种突变不会影响整体 Nef 折叠。这些发现支持 Nef 利用二聚复合物 X 射线晶体结构中存在的结构特征在脂质双层上同二聚化的内在能力。

































 京公网安备 11010802027423号
京公网安备 11010802027423号