Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Nanotherapy of Octanoic Acid Ameliorates Cardiac Arrest/Cardiopulmonary Resuscitation-Induced Brain Injury via RVG29- and Neutrophil Membrane-Mediated Injury Relay Targeting
ACS Nano ( IF 15.8 ) Pub Date : 2023-02-09 , DOI: 10.1021/acsnano.2c09931 Jingyuan Yang 1 , Pan Wang 2 , Xiangkang Jiang 1 , Jiefeng Xu 1 , Minhai Zhang 3 , Fei Liu 1 , Yao Lin 1 , Jiawei Tao 1 , Jiantao He 1 , Xing Zhou 2 , Mao Zhang 1
ACS Nano ( IF 15.8 ) Pub Date : 2023-02-09 , DOI: 10.1021/acsnano.2c09931 Jingyuan Yang 1 , Pan Wang 2 , Xiangkang Jiang 1 , Jiefeng Xu 1 , Minhai Zhang 3 , Fei Liu 1 , Yao Lin 1 , Jiawei Tao 1 , Jiantao He 1 , Xing Zhou 2 , Mao Zhang 1
Affiliation
|
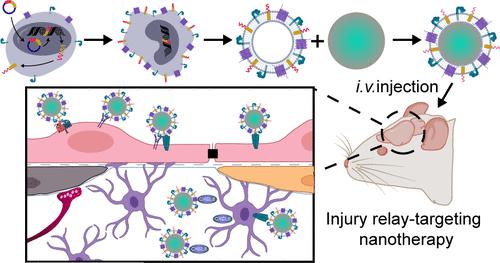
Treatment of cardiac arrest/cardiopulmonary resuscitation (CA/CPR)-induced brain injury remains a challenging issue without viable therapeutic options. Octanoic acid (OA), a lipid oil that is mainly metabolized in the astrocytes of the brain, is a promising treatment for this type of injury owing to its potential functions against oxidative stress, apoptosis, inflammation, and ability to stabilize mitochondria. However, the application of OA is strictly limited by its short half-life and low available concentration in the target organ. Herein, based on our previous research, an OA-based nanotherapy coated with a neutrophil membrane highly expressing RVG29, RVG29-H-NPOA, was successfully constructed by computer simulation-guided supramolecular assembly of polyethylenimine and OA. The in vitro and in vivo experiments showed that RVG29-H-NPOA could target and be distributed in the injured brain focus via the relay-targeted delivery mediated by RVG29-induced blood–brain barrier (BBB) penetration and neutrophil membrane protein-induced BBB binding and injury targeting. This results in enhancements of the antioxidant, antiapoptotic, mitochondrial stability-promoting and anti-inflammatory effects of OA and exhibited systematic alleviation of astrocyte injury, neuronal damage, and inflammatory response in the brain. Due to their systematic intervention in multiple pathological processes, RVG29-H-NPOA significantly increased the 24 h survival rate of CA/CPR model rats from 40% to 100% and significantly improved their neurological functions. Thus, RVG29-H-NPOA are expected to be a promising therapeutic for the treatment of CA/CPR-induced brain injury.
中文翻译:

辛酸的纳米疗法通过 RVG29 和中性粒细胞膜介导的损伤中继靶向改善心脏骤停/心肺复苏引起的脑损伤
心脏骤停/心肺复苏 (CA/CPR) 引起的脑损伤的治疗仍然是一个具有挑战性的问题,没有可行的治疗选择。辛酸 (OA) 是一种主要在大脑星形胶质细胞中代谢的脂质油,由于其潜在的抗氧化应激、细胞凋亡、炎症和稳定线粒体的能力,因此是一种很有前途的治疗此类损伤的方法。然而,OA的应用受到其半衰期短和靶器官可用浓度低的严格限制。在此,基于我们之前的研究,通过计算机模拟引导的聚乙烯亚胺和 OA 的超分子组装成功构建了一种基于 OA 的纳米疗法,该纳米疗法涂有高表达 RVG29 的中性粒细胞膜,RVG29-H-NP OA 。体外_和体内实验表明,RVG29-H-NP OA可以通过RVG29 诱导的血脑屏障 (BBB) 穿透和中性粒细胞膜蛋白诱导的 BBB 结合介导的中继靶向递送靶向并分布在受伤的脑病灶中,伤害瞄准。这导致 OA 的抗氧化、抗细胞凋亡、线粒体稳定性促进和抗炎作用增强,并表现出系统地减轻星形胶质细胞损伤、神经元损伤和大脑中的炎症反应。由于它们对多种病理过程的系统干预,RVG29-H-NP OACA/CPR 模型大鼠的 24 h 存活率从 40% 显着提高到 100%,并显着改善其神经功能。因此,RVG29-H-NP OA有望成为治疗 CA/CPR 诱发的脑损伤的有前途的治疗药物。
更新日期:2023-02-09
中文翻译:

辛酸的纳米疗法通过 RVG29 和中性粒细胞膜介导的损伤中继靶向改善心脏骤停/心肺复苏引起的脑损伤
心脏骤停/心肺复苏 (CA/CPR) 引起的脑损伤的治疗仍然是一个具有挑战性的问题,没有可行的治疗选择。辛酸 (OA) 是一种主要在大脑星形胶质细胞中代谢的脂质油,由于其潜在的抗氧化应激、细胞凋亡、炎症和稳定线粒体的能力,因此是一种很有前途的治疗此类损伤的方法。然而,OA的应用受到其半衰期短和靶器官可用浓度低的严格限制。在此,基于我们之前的研究,通过计算机模拟引导的聚乙烯亚胺和 OA 的超分子组装成功构建了一种基于 OA 的纳米疗法,该纳米疗法涂有高表达 RVG29 的中性粒细胞膜,RVG29-H-NP OA 。体外_和体内实验表明,RVG29-H-NP OA可以通过RVG29 诱导的血脑屏障 (BBB) 穿透和中性粒细胞膜蛋白诱导的 BBB 结合介导的中继靶向递送靶向并分布在受伤的脑病灶中,伤害瞄准。这导致 OA 的抗氧化、抗细胞凋亡、线粒体稳定性促进和抗炎作用增强,并表现出系统地减轻星形胶质细胞损伤、神经元损伤和大脑中的炎症反应。由于它们对多种病理过程的系统干预,RVG29-H-NP OACA/CPR 模型大鼠的 24 h 存活率从 40% 显着提高到 100%,并显着改善其神经功能。因此,RVG29-H-NP OA有望成为治疗 CA/CPR 诱发的脑损伤的有前途的治疗药物。






























 京公网安备 11010802027423号
京公网安备 11010802027423号