当前位置:
X-MOL 学术
›
Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rock–Fluid Interactions of Alkyl Ether Carboxylate Surfactants with Carbonates: Wettability Alteration, ζ-Potential, and Adsorption
Energy & Fuels ( IF 5.2 ) Pub Date : 2023-02-09 , DOI: 10.1021/acs.energyfuels.2c04099 Alexandra Scerbacova 1, 2 , Elena Kozlova 1 , Ahmed Barifcani 2 , Chi Minh Phan 2 , Tagir Karamov 1 , Alexey Cheremisin 1
Energy & Fuels ( IF 5.2 ) Pub Date : 2023-02-09 , DOI: 10.1021/acs.energyfuels.2c04099 Alexandra Scerbacova 1, 2 , Elena Kozlova 1 , Ahmed Barifcani 2 , Chi Minh Phan 2 , Tagir Karamov 1 , Alexey Cheremisin 1
Affiliation
|
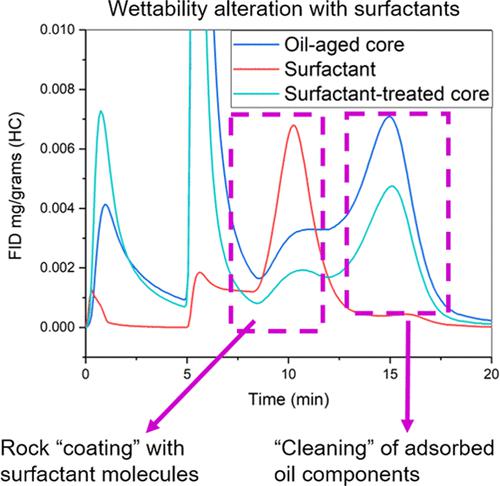
This work evaluated the rock–fluid interactions of two linear alkyl ether carboxylate (AEC) surfactants C11E11A and C12E7A in carbonates: wetting ability, ζ-potential, and adsorption capacity. Contact angle measurements showed that the ability of AECs to hydrophilize the surface is strongly affected by the ethylene oxide (EO) chain length and the presence of electrolytes. An AEC with a shorter EO chain C12E7A demonstrated a better wetting ability and decreased the contact angle value on the limestone surface from 113 to 27 ± 11° in deionized (DI) water and until 17 ± 5° in 5 wt % NaCl. The mechanism of surfactant action was assessed through thermodesorbed hydrocarbon analysis and organic matter characterization with the help of the Rock-Eval (RE) pyrolysis technique for the first time. The analysis results revealed that all surfactant compositions partially removed free oil and heavy oil components from limestone samples. Also, the presence of C12E7A molecules was detected in the samples after surfactant treatment that indicates that a better wetting ability is achieved by the combination of washing hydrocarbons and adsorption of surfactants on clean sites. The combination of ζ-potential measurements and static adsorption test on crushed limestone demonstrated that the adsorption of AECs in deionized water is not governed by electrostatic interactions and mainly occurs due to hydrogen bonding. Salinity has a significant effect on the adsorption behavior of AECs and leads to the partial destruction of hydrogen bonds and the shift from monomolecular adsorption in DI water to polymolecular adsorption in brines. The adsorption of C11E11A achieved ∼9 mg/g-rock and was higher than that of C12E7A, showing a maximum value of ∼4 mg/g-rock. This work provides a set of experiments that characterize the behavior of AECs during interactions with carbonate rock and describes a novel approach of the wettability shift mechanism assessment through RE pyrolysis.
中文翻译:

烷基醚羧酸盐表面活性剂与碳酸盐的岩石-流体相互作用:润湿性改变、ζ-电位和吸附
这项工作评估了两种直链烷基醚羧酸盐 (AEC) 表面活性剂 C 11 E 11 A 和 C 12 E 7 A 在碳酸盐岩中的岩石-流体相互作用:润湿能力、ζ 电位和吸附能力。接触角测量表明,AEC 使表面亲水化的能力受到环氧乙烷 (EO) 链长和电解质存在的强烈影响。具有较短 EO 链的 AEC C 12 E 7A 表现出更好的润湿能力,并且在去离子 (DI) 水中将石灰石表面的接触角值从 113 降低到 27 ± 11°,在 5 wt% NaCl 中降低到 17 ± 5°。首次借助 Rock-Eval (RE) 热解技术,通过热解吸碳氢化合物分析和有机物表征来评估表面活性剂的作用机制。分析结果表明,所有表面活性剂组合物都部分去除了石灰石样品中的游离油和重油成分。此外,C 12 E 7的存在在表面活性剂处理后的样品中检测到一个分子,表明通过洗涤碳氢化合物和表面活性剂在清洁部位的吸附相结合,可以获得更好的润湿能力。结合 ζ 电位测量和碎石灰石静态吸附试验表明,AECs 在去离子水中的吸附不受静电相互作用的控制,主要是由于氢键作用而发生的。盐度对 AEC 的吸附行为有显着影响,并导致氢键的部分破坏以及从去离子水中的单分子吸附转变为盐水中的多分子吸附。C 11 E 11 A的吸附量达到~9 mg/g-rock,高于 C 12 E 7A,最大值为 ∼4 mg/g-rock。这项工作提供了一组实验来表征 AEC 在与碳酸盐岩相互作用期间的行为,并描述了一种通过稀土热解评估润湿性转变机制的新方法。
更新日期:2023-02-09
中文翻译:

烷基醚羧酸盐表面活性剂与碳酸盐的岩石-流体相互作用:润湿性改变、ζ-电位和吸附
这项工作评估了两种直链烷基醚羧酸盐 (AEC) 表面活性剂 C 11 E 11 A 和 C 12 E 7 A 在碳酸盐岩中的岩石-流体相互作用:润湿能力、ζ 电位和吸附能力。接触角测量表明,AEC 使表面亲水化的能力受到环氧乙烷 (EO) 链长和电解质存在的强烈影响。具有较短 EO 链的 AEC C 12 E 7A 表现出更好的润湿能力,并且在去离子 (DI) 水中将石灰石表面的接触角值从 113 降低到 27 ± 11°,在 5 wt% NaCl 中降低到 17 ± 5°。首次借助 Rock-Eval (RE) 热解技术,通过热解吸碳氢化合物分析和有机物表征来评估表面活性剂的作用机制。分析结果表明,所有表面活性剂组合物都部分去除了石灰石样品中的游离油和重油成分。此外,C 12 E 7的存在在表面活性剂处理后的样品中检测到一个分子,表明通过洗涤碳氢化合物和表面活性剂在清洁部位的吸附相结合,可以获得更好的润湿能力。结合 ζ 电位测量和碎石灰石静态吸附试验表明,AECs 在去离子水中的吸附不受静电相互作用的控制,主要是由于氢键作用而发生的。盐度对 AEC 的吸附行为有显着影响,并导致氢键的部分破坏以及从去离子水中的单分子吸附转变为盐水中的多分子吸附。C 11 E 11 A的吸附量达到~9 mg/g-rock,高于 C 12 E 7A,最大值为 ∼4 mg/g-rock。这项工作提供了一组实验来表征 AEC 在与碳酸盐岩相互作用期间的行为,并描述了一种通过稀土热解评估润湿性转变机制的新方法。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号