当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Propane Dehydrogenation on PtxSny (x, y ≤ 4) Clusters on Al2O3(110)
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-02-09 , DOI: 10.1021/acscatal.2c05671
Yilang Liu 1 , Xue Zong 1, 2 , Abhirup Patra 1 , Stavros Caratzoulas 1 , Dionisios G. Vlachos 1, 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-02-09 , DOI: 10.1021/acscatal.2c05671
Yilang Liu 1 , Xue Zong 1, 2 , Abhirup Patra 1 , Stavros Caratzoulas 1 , Dionisios G. Vlachos 1, 2
Affiliation
|
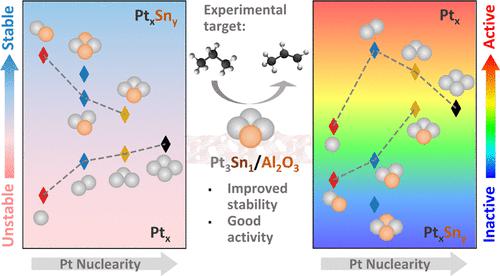
With increasing interest in atomically dispersed and sub-nanometer Pt and Pt–Sn cluster catalysts for propane dehydrogenation (PDH), understanding structure–property relationships is of great importance. In this work, we present density functional theory calculations and microkinetic modeling of PDH on eight alumina-supported Ptx (x = 1–4) and PtxSny (x = 1–3, y = 1 and x = 2, y = 2) model catalysts and elucidate the effects of Pt nuclearity and Sn heteroatoms. Ptx clusters are prone to sintering, and their stability is improved with the addition of Sn. The Ptx activity changes nonmonotonically with cluster size. Pt1 presents the most favorable dehydrogenation barriers, but its PDH rate is highly limited by the blocking of sites by hydrogen. A volcano-like activity–nuclearity relation is found, with Pt2 being the most active cluster. An increase in Pt nuclearity beyond x = 2 leads to lower rates due to increased dehydrogenation barriers. Moderate addition of Sn somewhat depresses the Pt activity, whereas PtxSny clusters with a Pt/Sn ratio of 1:1 become inactive. The Pt3Sn1 cluster exhibits good activity with improved stability compared to Pt3 and Pt2 and could be an experimental target. While Sn promotes propylene desorption, propane hydrogenolysis to C1 and C2 species on single atoms and small Ptx and PtxSny clusters is unfavorable. Low selectivity to propylene seen experimentally should instead stem from other routes.
更新日期:2023-02-09




































 京公网安备 11010802027423号
京公网安备 11010802027423号