当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical Understanding of Potential-Dependent Electrocatalytic CO2RR and Competition with HER upon Cobalt Single Atom Supported by Phthalocyanine Monolayer
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2023-02-07 , DOI: 10.1021/acs.jpcc.2c08319 Tao Wang 1 , Long Zhou 1 , Shuwei Xia 1, 2 , Liangmin Yu 1, 2
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2023-02-07 , DOI: 10.1021/acs.jpcc.2c08319 Tao Wang 1 , Long Zhou 1 , Shuwei Xia 1, 2 , Liangmin Yu 1, 2
Affiliation
|
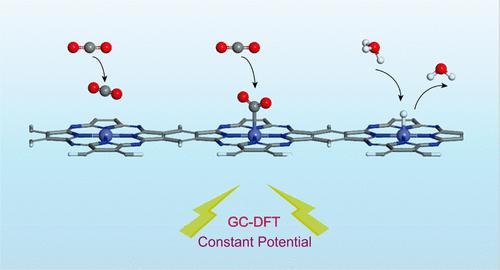
Cobalt-doped phthalocyanine monolayer (Co-Pc) catalyst has shown promising performance in electrochemical CO2 reduction experimentally. However, most theoretic investigations based on computational hydrogen electrode (CHE) model inaccurately predict catalytic behaviors and exhibit contradictive mechanistic pathways. In this work, grand canonical density functional theory (GC-DFT) combined CANDLE continuum solvation model was used to more precisely simulate CO2RR upon Co-Pc. The calculation results reasonably explained the activation of CO2 and HER (hydrogen evolution reaction) competition and also predicted potential determined step and onset potential, which all are excellently consistent with experimental values. The potential-dependent competition among physical and chemical adsorption of CO2 and H formation and the reason for high CO2RR selectivity at a specific potential range were well explained: the charge of the reaction intermediate is the key to potential-dependent behaviors; larger electron transfer in H adsorption than CO2 adsorption is the primary reason for HER suppression of CO2RR at high potential. This is a general phenomenon seen in various heterogeneous catalysts, hindering practical electrochemical CO2RR.
中文翻译:

电位依赖性电催化 CO2RR 的理论理解以及与 HER 在酞菁单层负载的钴单原子上的竞争
钴掺杂酞菁单层 (Co-Pc) 催化剂在电化学 CO 2还原实验中表现出良好的性能。然而,大多数基于计算氢电极 (CHE) 模型的理论研究都不能准确预测催化行为,并表现出相互矛盾的机制途径。在这项工作中,使用大正则密度泛函理论 (GC-DFT) 结合 CANDLE 连续介质溶剂化模型来更精确地模拟 Co-Pc 上的 CO 2 RR。计算结果合理解释了CO 2的活化和 HER(析氢反应)竞争,还预测了电位确定步骤和起始电位,这些都与实验值非常一致。CO 2的物理和化学吸附与H形成之间的电位依赖竞争以及在特定电位范围内CO 2 RR选择性高的原因得到了很好的解释:反应中间体的电荷是电位依赖行为的关键;H 吸附中比 CO 2吸附中更大的电子转移是 HER在高电位下抑制 CO 2 RR的主要原因。这是在各种非均相催化剂中常见的现象,阻碍了实际的电化学 CO 2 RR。
更新日期:2023-02-07
中文翻译:

电位依赖性电催化 CO2RR 的理论理解以及与 HER 在酞菁单层负载的钴单原子上的竞争
钴掺杂酞菁单层 (Co-Pc) 催化剂在电化学 CO 2还原实验中表现出良好的性能。然而,大多数基于计算氢电极 (CHE) 模型的理论研究都不能准确预测催化行为,并表现出相互矛盾的机制途径。在这项工作中,使用大正则密度泛函理论 (GC-DFT) 结合 CANDLE 连续介质溶剂化模型来更精确地模拟 Co-Pc 上的 CO 2 RR。计算结果合理解释了CO 2的活化和 HER(析氢反应)竞争,还预测了电位确定步骤和起始电位,这些都与实验值非常一致。CO 2的物理和化学吸附与H形成之间的电位依赖竞争以及在特定电位范围内CO 2 RR选择性高的原因得到了很好的解释:反应中间体的电荷是电位依赖行为的关键;H 吸附中比 CO 2吸附中更大的电子转移是 HER在高电位下抑制 CO 2 RR的主要原因。这是在各种非均相催化剂中常见的现象,阻碍了实际的电化学 CO 2 RR。































 京公网安备 11010802027423号
京公网安备 11010802027423号