当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Terminal Hydride of Hafnium Supported by a Triamidoamine Ligand
Organometallics ( IF 2.5 ) Pub Date : 2023-02-08 , DOI: 10.1021/acs.organomet.2c00566 Sebastian Schrader 1 , Priyabrata Ghana 1 , Alexander Hoffmann 1 , Thomas P. Spaniol 1 , Jun Okuda 1
Organometallics ( IF 2.5 ) Pub Date : 2023-02-08 , DOI: 10.1021/acs.organomet.2c00566 Sebastian Schrader 1 , Priyabrata Ghana 1 , Alexander Hoffmann 1 , Thomas P. Spaniol 1 , Jun Okuda 1
Affiliation
|
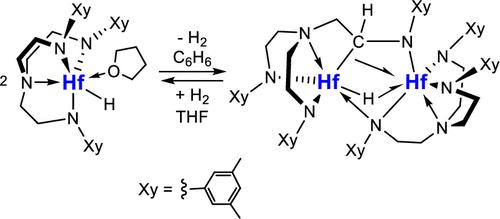
A mononuclear hafnium hydride supported by an aryl-substituted triamidoamine ligand [HfH(thf)(Xy-N3N)] (Xy-N3N = {(3,5-Me2C6H3)NCH2CH2}3N3–) was synthesized by either hydrogenolysis of the hydrocarbyl complex [Hf(R)(thf)(Xy-N3N)] (R = Me, CH2SiMe3, η3-C3H5, CH2Ph) in tetrahydrofuran (THF) or, more conveniently, by salt metathesis of the chloro precursor [HfCl(thf)(Xy-N3N)] with NaBEt3H in methylcyclohexane. The structure of a terminal hydride with a distorted capped trigonal bipyramidal geometry was characterized by single crystal X-ray diffraction (d(Hf–H): 1.83(3) Å) and by 1H NMR spectroscopy. The unusually low field shifted 1H chemical shift for the hydride ligand was detected at δ 18.28 ppm. Dehydrogenative thermolysis of the hydride complex at 25 °C in benzene or toluene resulted in deprotonation of the α-hydrogen of one of the three XyNCH2CH2 groups in the ligand by the hydride, followed by the combination of this deprotonated anionic complex with cationic complex [Hf(Xy-N3N)]+ to give an unsymmetric dinuclear complex bridged by μ-H, μ-NXy, and μ,κN,κC-XyNCHCH2 groups. This decomposition product was characterized by single crystal X-ray diffraction and by 1H and 13C NMR spectroscopy and analyzed by density functional theory (DFT) calculations. Hydrogenation in THF at 25 °C reformed the hydride complex over a period of 48 h. The hydride complex reacted with olefins RHC═CH2 (R = H, Et, nHex, cHex, Ph) to give the corresponding anti-Markovnikov insertion products [Hf(CH2CH2R)(thf)n(Xy-N3N)]. Catalytic hydrogenation of olefins was achieved with 5 mol % of the hydride complex at 70 °C in THF or benzene.
中文翻译:

由三氨基胺配体支持的铪末端氢化物
由芳基取代的三氨基胺配体 [HfH(thf)(Xy-N 3 N)] (Xy-N 3 N = {(3,5-Me 2 C 6 H 3 )NCH 2 CH 2 } 3 N 3– ) 通过烃基络合物 [Hf(R)(thf)(Xy-N 3 N)] (R = Me, CH 2 SiMe 3 , η 3 -C 3 H 5 , CH 2 Ph) 在四氢呋喃 (THF) 中,或者更方便地,通过氯前体 [HfCl(thf)(Xy-N 3 N)] 与 NaBEt 3的盐置换H 在甲基环己烷中。通过单晶 X 射线衍射 ( d (Hf–H): 1.83(3) Å) 和1 H NMR 光谱表征具有扭曲封端三角双锥几何结构的末端氢化物的结构。在 δ 18.28 ppm 处检测到氢化物配体异常低的场位移1 H 化学位移。氢化物配合物在 25 °C 下在苯或甲苯中的脱氢热解导致配体中三个 XyNCH 2 CH 2基团之一的 α-氢被氢化物去质子化,随后这种去质子化的阴离子配合物与阳离子结合复数 [Hf(Xy-N 3 N)] +给出由 μ-H、μ-NXy 和 μ,κN,κC-XyNCHCH 2基团桥接的不对称双核复合物。该分解产物通过单晶 X 射线衍射和1 H 和13 C NMR 光谱进行表征,并通过密度泛函理论 (DFT) 计算进行分析。在 THF 中于 25 °C 氢化 48 小时后重新形成氢化物络合物。氢化物络合物与烯烃RHC=CH 2 (R = H, Et, n Hex, c Hex, Ph)反应得到相应的反马尔可夫尼科夫插入产物[Hf(CH 2 CH 2 R)(thf) n (Xy- 3 _N)]。烯烃的催化氢化是在 70 °C 的 THF 或苯中用 5 mol% 的氢化物络合物实现的。
更新日期:2023-02-08
中文翻译:

由三氨基胺配体支持的铪末端氢化物
由芳基取代的三氨基胺配体 [HfH(thf)(Xy-N 3 N)] (Xy-N 3 N = {(3,5-Me 2 C 6 H 3 )NCH 2 CH 2 } 3 N 3– ) 通过烃基络合物 [Hf(R)(thf)(Xy-N 3 N)] (R = Me, CH 2 SiMe 3 , η 3 -C 3 H 5 , CH 2 Ph) 在四氢呋喃 (THF) 中,或者更方便地,通过氯前体 [HfCl(thf)(Xy-N 3 N)] 与 NaBEt 3的盐置换H 在甲基环己烷中。通过单晶 X 射线衍射 ( d (Hf–H): 1.83(3) Å) 和1 H NMR 光谱表征具有扭曲封端三角双锥几何结构的末端氢化物的结构。在 δ 18.28 ppm 处检测到氢化物配体异常低的场位移1 H 化学位移。氢化物配合物在 25 °C 下在苯或甲苯中的脱氢热解导致配体中三个 XyNCH 2 CH 2基团之一的 α-氢被氢化物去质子化,随后这种去质子化的阴离子配合物与阳离子结合复数 [Hf(Xy-N 3 N)] +给出由 μ-H、μ-NXy 和 μ,κN,κC-XyNCHCH 2基团桥接的不对称双核复合物。该分解产物通过单晶 X 射线衍射和1 H 和13 C NMR 光谱进行表征,并通过密度泛函理论 (DFT) 计算进行分析。在 THF 中于 25 °C 氢化 48 小时后重新形成氢化物络合物。氢化物络合物与烯烃RHC=CH 2 (R = H, Et, n Hex, c Hex, Ph)反应得到相应的反马尔可夫尼科夫插入产物[Hf(CH 2 CH 2 R)(thf) n (Xy- 3 _N)]。烯烃的催化氢化是在 70 °C 的 THF 或苯中用 5 mol% 的氢化物络合物实现的。

































 京公网安备 11010802027423号
京公网安备 11010802027423号