当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Production of Dichloroacetonitrile from Derivatives of Isoxaflutole Herbicide during Water Treatment
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2023-02-07 , DOI: 10.1021/acs.est.2c06376 Jacqueline Rogers 1 , Moshan Chen 1 , Kaichao Yang 1 , Jonathan Graham 1 , Kimberly M Parker 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2023-02-07 , DOI: 10.1021/acs.est.2c06376 Jacqueline Rogers 1 , Moshan Chen 1 , Kaichao Yang 1 , Jonathan Graham 1 , Kimberly M Parker 1
Affiliation
|
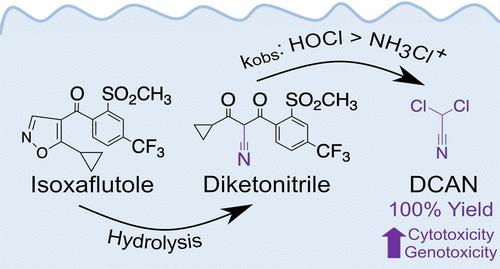
The herbicide isoxaflutole has the potential to contaminate drinking water directly, as well as upon hydrolyzing to its active form diketonitrile. Diketonitrile also may impact water quality by acting as a precursor for dichloroacetonitrile (DCAN), which is an unregulated but highly toxic disinfection byproduct (DBP). In this study, we investigated the reaction of diketonitrile with free chlorine and chloramine to form DCAN. We found that diketonitrile reacts with free chlorine within seconds but reacts with chloramine on the time scale of hours to days. In the presence of both oxidants, DCAN was generated at yields up to 100%. Diketonitrile reacted fastest with chlorine at circumneutral pH, which was consistent with base-catalyzed halogenation involving the enolate form of diketonitrile present at alkaline pH and electrophilic hypochlorous acid, which decreases in abundance above its pKa (7.5). In contrast, we found that diketonitrile reacts faster with chloramine as pH values decreased, consistent with an attack on the enolate by electrophilic protonated monochloramine that increases in abundance at acidic pH approaching its pKa (1.6). Our results indicate that increasing isoxaflutole use, particularly in light of the recent release of genetically modified isoxaflutole-tolerant crops, could result in greater occurrences of a high-yield DCAN precursor during disinfection.
中文翻译:

水处理过程中异恶唑草酮衍生物生产二氯乙腈
除草剂异恶唑草酮有可能直接污染饮用水,以及水解成活性形式二酮腈后也可能污染饮用水。二酮腈还可能通过作为二氯乙腈 (DCAN) 的前体来影响水质,二氯乙腈 (DCAN) 是一种不受管制但剧毒的消毒副产品 (DBP)。在本研究中,我们研究了二酮腈与游离氯和氯胺形成 DCAN 的反应。我们发现二酮腈在几秒钟内与游离氯反应,但与氯胺反应需要几小时到几天的时间。在两种氧化剂存在下,DCAN 的产率高达 100%。二酮腈在中性pH值下与氯反应最快,这与碱催化卤化反应一致,涉及碱性 pH 值下存在的烯醇化形式的二酮腈和亲电子次氯酸,其丰度在高于其 p Ka (7.5) 时减少。相比之下,我们发现,随着 pH 值降低,二酮腈与氯胺的反应速度更快,这与亲电质子化一氯胺对烯醇化物的攻击一致,在酸性 pH 值接近其 pKa (1.6) 时,亲电质子化一氯胺的丰度会增加。我们的结果表明,增加异恶唑草酮的使用,特别是考虑到最近释放的转基因耐异恶唑草酮作物,可能会导致消毒过程中更多地出现高产 DCAN 前体。
更新日期:2023-02-07
中文翻译:

水处理过程中异恶唑草酮衍生物生产二氯乙腈
除草剂异恶唑草酮有可能直接污染饮用水,以及水解成活性形式二酮腈后也可能污染饮用水。二酮腈还可能通过作为二氯乙腈 (DCAN) 的前体来影响水质,二氯乙腈 (DCAN) 是一种不受管制但剧毒的消毒副产品 (DBP)。在本研究中,我们研究了二酮腈与游离氯和氯胺形成 DCAN 的反应。我们发现二酮腈在几秒钟内与游离氯反应,但与氯胺反应需要几小时到几天的时间。在两种氧化剂存在下,DCAN 的产率高达 100%。二酮腈在中性pH值下与氯反应最快,这与碱催化卤化反应一致,涉及碱性 pH 值下存在的烯醇化形式的二酮腈和亲电子次氯酸,其丰度在高于其 p Ka (7.5) 时减少。相比之下,我们发现,随着 pH 值降低,二酮腈与氯胺的反应速度更快,这与亲电质子化一氯胺对烯醇化物的攻击一致,在酸性 pH 值接近其 pKa (1.6) 时,亲电质子化一氯胺的丰度会增加。我们的结果表明,增加异恶唑草酮的使用,特别是考虑到最近释放的转基因耐异恶唑草酮作物,可能会导致消毒过程中更多地出现高产 DCAN 前体。

































 京公网安备 11010802027423号
京公网安备 11010802027423号