当前位置:
X-MOL 学术
›
J. Am. Soc. Mass Spectrom.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Proteomic Profiling of Cerebrospinal Fluids to Reveal Novel Conformational Biomarkers for Alzheimer’s Disease
Journal of the American Society for Mass Spectrometry ( IF 3.1 ) Pub Date : 2023-02-06 , DOI: 10.1021/jasms.2c00332
Bin Wang 1 , Xiaofang Zhong 1 , Lauren Fields 2 , Haiyan Lu 1 , Zexin Zhu 1 , Lingjun Li 1, 2, 3, 4
Journal of the American Society for Mass Spectrometry ( IF 3.1 ) Pub Date : 2023-02-06 , DOI: 10.1021/jasms.2c00332
Bin Wang 1 , Xiaofang Zhong 1 , Lauren Fields 2 , Haiyan Lu 1 , Zexin Zhu 1 , Lingjun Li 1, 2, 3, 4
Affiliation
|
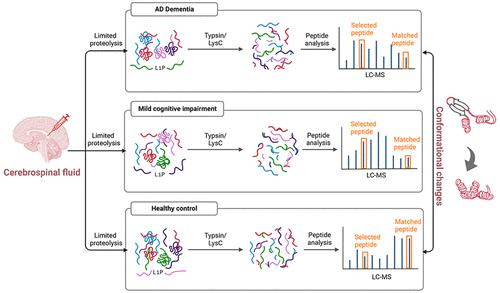
Alzheimer’s disease (AD) is the most common representation of dementia, with brain pathological hallmarks of protein abnormal aggregation, such as with amyloid beta and tau protein. It is well established that posttranslational modifications on tau protein, particularly phosphorylation, increase the likelihood of its aggregation and subsequent formation of neurofibrillary tangles, another hallmark of AD. As additional misfolded proteins presumably exist distinctly in AD disease states, which would serve as potential source of AD biomarkers, we used limited proteolysis-coupled with mass spectrometry (LiP-MS) to probe protein structural changes. After optimizing the LiP-MS conditions, we further applied this method to human cerebrospinal fluid specimens collected from healthy control, mild cognitive impairment (MCI), and AD subject groups to characterize proteome-wide misfolding tendencies as a result of disease progression. The fully tryptic peptides embedding LiP sites were compared with the half-tryptic peptides generated from internal cleavage of the same region to determine any structural unfolding or misfolding. We discovered hundreds of significantly up- and down-regulated peptides associated with MCI and AD indicating their potential structural changes in AD progression. Moreover, we detected 53 structurally changed regions in 12 proteins with high confidence between the healthy control and disease groups, illustrating the functional relevance of these proteins with AD progression. These newly discovered conformational biomarker candidates establish valuable future directions for exploring the molecular mechanism of designing therapeutic targets for AD.
中文翻译:

脑脊液结构蛋白质组学分析揭示阿尔茨海默氏病的新型构象生物标志物
阿尔茨海默氏病 (AD) 是痴呆症最常见的表现形式,具有蛋白质异常聚集的脑病理特征,例如淀粉样蛋白和 tau 蛋白。众所周知,tau 蛋白的翻译后修饰,特别是磷酸化,增加了其聚集和随后形成神经原纤维缠结的可能性,这是 AD 的另一个标志。由于其他错误折叠的蛋白质可能明显存在于 AD 疾病状态中,这将作为 AD 生物标志物的潜在来源,我们使用有限的蛋白水解与质谱 (LiP-MS) 相结合来探测蛋白质结构变化。在优化 LiP-MS 条件后,我们进一步将该方法应用于从健康对照、轻度认知障碍 (MCI)、和 AD 主题组来描述疾病进展导致的蛋白质组范围内的错误折叠趋势。将嵌入 LiP 位点的完全胰蛋白酶肽与同一区域内部裂解产生的半胰蛋白酶肽进行比较,以确定任何结构展开或错误折叠。我们发现了数百种与 MCI 和 AD 相关的显着上调和下调的肽,表明它们在 AD 进展中的潜在结构变化。此外,我们在 12 种蛋白质中检测到 53 个结构改变的区域,在健康对照组和疾病组之间具有很高的置信度,说明了这些蛋白质与 AD 进展的功能相关性。
更新日期:2023-02-06
中文翻译:

脑脊液结构蛋白质组学分析揭示阿尔茨海默氏病的新型构象生物标志物
阿尔茨海默氏病 (AD) 是痴呆症最常见的表现形式,具有蛋白质异常聚集的脑病理特征,例如淀粉样蛋白和 tau 蛋白。众所周知,tau 蛋白的翻译后修饰,特别是磷酸化,增加了其聚集和随后形成神经原纤维缠结的可能性,这是 AD 的另一个标志。由于其他错误折叠的蛋白质可能明显存在于 AD 疾病状态中,这将作为 AD 生物标志物的潜在来源,我们使用有限的蛋白水解与质谱 (LiP-MS) 相结合来探测蛋白质结构变化。在优化 LiP-MS 条件后,我们进一步将该方法应用于从健康对照、轻度认知障碍 (MCI)、和 AD 主题组来描述疾病进展导致的蛋白质组范围内的错误折叠趋势。将嵌入 LiP 位点的完全胰蛋白酶肽与同一区域内部裂解产生的半胰蛋白酶肽进行比较,以确定任何结构展开或错误折叠。我们发现了数百种与 MCI 和 AD 相关的显着上调和下调的肽,表明它们在 AD 进展中的潜在结构变化。此外,我们在 12 种蛋白质中检测到 53 个结构改变的区域,在健康对照组和疾病组之间具有很高的置信度,说明了这些蛋白质与 AD 进展的功能相关性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号