当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanical Understanding of Li–CO2 Batteries: The Critical Role of Forming Intermediate *Li2O
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2023-02-07 , DOI: 10.1021/acs.jpclett.3c00060 Xinxin Zhang 1 , Yu Wang 1 , Yafei Li 1
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2023-02-07 , DOI: 10.1021/acs.jpclett.3c00060 Xinxin Zhang 1 , Yu Wang 1 , Yafei Li 1
Affiliation
|
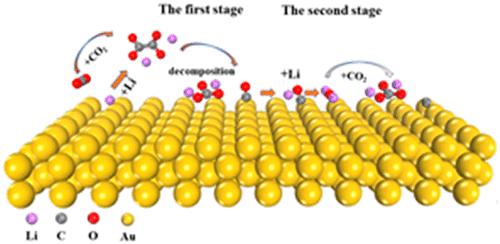
The emerging Li–CO2 batteries are considered a promising next-generation power system because they can fix CO2 while storing energy; however, their underlying mechanism remains elusive, impeding their efficient development. Meanwhile, apart from the conventional discharge product Li2CO3, the unexpected Li2O species has also been detected, but its formation process is thus far undecided. Here, we report a new mechanism for Li–CO2 batteries using first-principles calculations, which explains the long-standing puzzles. We show that such a process can be divided into two stages: (I) forming intermediate *Li2C2O4 via surface lithiation and (II) generating −Li2CO3 and C through a *Li2O-mediated pathway. We discover that the major kinetic barrier occurs in the coupling of *Li2CO2 and CO2 in the first stage. Especially, in the second stage, *CO produced from *Li2C2O4 decomposition is preferentially lithiated to *LiOC rather than disproportionated, and then *LiOC can be further lithiated to intermediate *Li2O after C nucleation, which contributes to the final formation of Li2CO3 in the presence of sufficient CO2.
中文翻译:

Li-CO2 电池的机械理解:形成中间体 *Li2O 的关键作用
新兴的 Li-CO 2电池被认为是有前途的下一代电力系统,因为它们可以在储存能量的同时固定 CO 2;然而,它们的潜在机制仍然难以捉摸,阻碍了它们的有效发展。同时,除了常规的放电产物Li 2 CO 3外,还检测到了意想不到的Li 2 O物种,但其形成过程至今未定。在这里,我们报告了一种使用第一性原理计算的Li-CO 2电池的新机制,它解释了长期存在的难题。我们证明这样的过程可以分为两个阶段:(I) 形成中间体 *Li 2 C 2 O 4通过表面锂化和 (II)通过*Li 2 O 介导的途径生成-Li 2 CO 3和C。我们发现主要的动力学势垒发生在第一阶段*Li 2 CO 2和CO 2的偶联中。特别是,在第二阶段,*Li 2 C 2 O 4分解产生的*CO优先锂化为*LiOC而不是歧化,然后*LiOC在C成核后可以进一步锂化为中间体*Li 2 O,这有助于在足够的 CO 2存在下最终形成 Li 2 CO 3。
更新日期:2023-02-07
中文翻译:

Li-CO2 电池的机械理解:形成中间体 *Li2O 的关键作用
新兴的 Li-CO 2电池被认为是有前途的下一代电力系统,因为它们可以在储存能量的同时固定 CO 2;然而,它们的潜在机制仍然难以捉摸,阻碍了它们的有效发展。同时,除了常规的放电产物Li 2 CO 3外,还检测到了意想不到的Li 2 O物种,但其形成过程至今未定。在这里,我们报告了一种使用第一性原理计算的Li-CO 2电池的新机制,它解释了长期存在的难题。我们证明这样的过程可以分为两个阶段:(I) 形成中间体 *Li 2 C 2 O 4通过表面锂化和 (II)通过*Li 2 O 介导的途径生成-Li 2 CO 3和C。我们发现主要的动力学势垒发生在第一阶段*Li 2 CO 2和CO 2的偶联中。特别是,在第二阶段,*Li 2 C 2 O 4分解产生的*CO优先锂化为*LiOC而不是歧化,然后*LiOC在C成核后可以进一步锂化为中间体*Li 2 O,这有助于在足够的 CO 2存在下最终形成 Li 2 CO 3。

































 京公网安备 11010802027423号
京公网安备 11010802027423号