当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Modulation of CO2 adsorption thermodynamics and selectivity in alkali-carbonate activated N-rich porous carbons
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2023-02-07 , DOI: 10.1039/d2ta09376f J. Ehren Eichler 1 , James N. Burrow 2 , Naman Katyal 1 , Graeme Henkelman 1 , C. Buddie Mullins 1, 2
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2023-02-07 , DOI: 10.1039/d2ta09376f J. Ehren Eichler 1 , James N. Burrow 2 , Naman Katyal 1 , Graeme Henkelman 1 , C. Buddie Mullins 1, 2
Affiliation
|
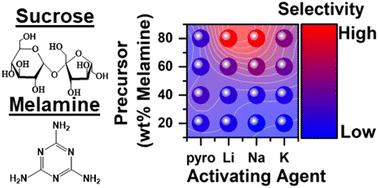
Here we analyze how changes in the charge density of activating alkali cations (lithium, sodium, and potassium) alters the synthesis and resulting physicochemical properties of N-rich activated carbons. In general, the synthesis reagents had significant influence on the total nitrogen content (2–24 at%), the chemical environment of the nitrogen species, the specific surface area (∼600–4300 m2 g−1), and the types of pores that formed in the activated materials. Each sample was screened for carbon dioxide (CO2) and nitrogen (N2) gas adsorption. From application of the ideal adsorbed solution theory, the predicted CO2/N2 selectivity spanned a large range from ∼8 to ∼150 at 15% CO2 and was dependent on d-spacing, surface N content, and porosity. Finally, the materials were analyzed with a simplified temperature swing adsorption model to estimate the optimal working capacity and regeneration energy of the materials in a cyclic process. Overall, this study demonstrates that while the precursor nitrogen content drives significant changes in the isotherm shape, a careful choice of activating cation during synthesis of advanced porous carbons can strongly influence physicochemical properties and the resulting thermodynamics and selectivity of CO2 adsorption.
中文翻译:

碱金属碳酸盐活化富氮多孔碳中 CO2 吸附热力学和选择性的调节
在这里,我们分析了活化碱金属阳离子(锂、钠和钾)的电荷密度变化如何改变富氮活性炭的合成和由此产生的物理化学性质。一般来说,合成试剂对总氮含量(2-24 at%)、氮物种的化学环境、比表面积(~600-4300 m 2 g -1)和类型有显着影响。活性材料中形成的孔隙。筛选每个样品的二氧化碳 (CO 2 ) 和氮气 (N 2 ) 气体吸附。根据理想吸附溶液理论的应用,在 15% CO 时,预测的 CO 2 /N 2选择性跨越了从 ∼8 到 ∼150 的大范围2并且取决于d间距、表面 N 含量和孔隙率。最后,使用简化的变温吸附模型对材料进行分析,以估算材料在循环过程中的最佳工作容量和再生能量。总的来说,这项研究表明,虽然前体氮含量会导致等温线形状发生显着变化,但在高级多孔碳的合成过程中仔细选择活化阳离子会强烈影响物理化学性质以及由此产生的热力学和 CO 2 吸附选择性。
更新日期:2023-02-07
中文翻译:

碱金属碳酸盐活化富氮多孔碳中 CO2 吸附热力学和选择性的调节
在这里,我们分析了活化碱金属阳离子(锂、钠和钾)的电荷密度变化如何改变富氮活性炭的合成和由此产生的物理化学性质。一般来说,合成试剂对总氮含量(2-24 at%)、氮物种的化学环境、比表面积(~600-4300 m 2 g -1)和类型有显着影响。活性材料中形成的孔隙。筛选每个样品的二氧化碳 (CO 2 ) 和氮气 (N 2 ) 气体吸附。根据理想吸附溶液理论的应用,在 15% CO 时,预测的 CO 2 /N 2选择性跨越了从 ∼8 到 ∼150 的大范围2并且取决于d间距、表面 N 含量和孔隙率。最后,使用简化的变温吸附模型对材料进行分析,以估算材料在循环过程中的最佳工作容量和再生能量。总的来说,这项研究表明,虽然前体氮含量会导致等温线形状发生显着变化,但在高级多孔碳的合成过程中仔细选择活化阳离子会强烈影响物理化学性质以及由此产生的热力学和 CO 2 吸附选择性。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号