Journal of Energy Chemistry ( IF 14.0 ) Pub Date : 2023-01-24 , DOI: 10.1016/j.jechem.2023.01.012 Kaixuan Ma , Gongzheng Yang , Chengxin Wang
|
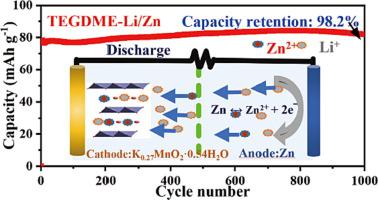
Aqueous rechargeable zinc-based batteries have attracted increasing interest and been considered potential alternatives for state-of-the-art lithium-ion batteries because of the low cost and high safety. Many cathode materials have been gradually developed and demonstrated excellent electrochemical performances. However, the complex electrochemistry, inevitable hydrogen release, and zinc corrosion severely hinder the practical application. The most concerned Zn-MnO2 batteries still suffer from the Mn dissolution and formation of byproducts. By adding organic solvents to inhibit the activity of water molecules, the hydrous organic electrolytes provide a sound solution for eliminating the unfavorable factors. Here we report a tetraethylene glycol dimethyl ether-based hydrous organic electrolyte consisting of LiClO4·3H2O and Zn(ClO4)2·6H2O, and a birnessite-type MnO2 cathode material for Zn-MnO2 batteries. The Li+/Zn2+ ions co-(de)insertion mechanism is ascertained by the structural and morphological analyses. The electrostatic interaction between inserted ions and crystal structure is reduced effectively by employment of monovalent Li+ ions, which ensures structural stability of cathode materials. Hydrous tetraglyme electrolyte inhibits the activity of water molecules and thus avoids the formation of byproduct Zn4ClO4(OH)7. Meanwhile, highly stable Zn plating/stripping for over 1500 h, an average coulombic efficiency of >99% in long-term cycling, and ultralong storage life (the cells can work well after stored over 1 year) are simultaneously realized in the novel electrolyte. Benefitting from these aspects, the Zn-MnO2 batteries manifest high specific capacity of 132 mA h g−1, an operating voltage of 1.25 V, and a capacity retention of >98% after 1000 cycles at a current density of 200 mA g−1.
中文翻译:

使用含水四甘醇二甲醚电解质的可储存和耐用的 Zn-MnO2 电池
由于低成本和高安全性,水系可充电锌基电池引起了越来越多的兴趣,并被认为是最先进锂离子电池的潜在替代品。许多正极材料已被逐步开发并表现出优异的电化学性能。然而,复杂的电化学、不可避免的氢释放和锌腐蚀严重阻碍了实际应用。最受关注的Zn-MnO 2电池仍然受到锰溶解和副产物形成的影响。通过添加有机溶剂抑制水分子的活性,含水有机电解质为消除不利因素提供了良好的解决方案。在这里,我们报道了一种基于四乙二醇二甲醚的含水有机电解质,由 LiClO 4 ·3H 2 O 和 Zn(ClO 4 ) 2 ·6H 2 O 组成,以及用于 Zn-MnO 2电池的水钠锰矿型 MnO 2正极材料。Li + /Zn 2+离子共(去)插入机制通过结构和形态分析确定。通过使用单价Li +离子,有效降低插入离子与晶体结构之间的静电相互作用,确保了正极材料的结构稳定性。含水四甘醇二甲醚电解质抑制水分子的活性,从而避免副产物 Zn 4 ClO 4 (OH) 7的形成。同时,该新型电解液同时实现了超过1500小时的高稳定性镀锌/退锌、长期循环平均库仑效率>99%和超长储存寿命(电池储存1年以上即可正常工作) . 受益于这些方面,Zn-MnO 2电池表现出 132 mA h g -1的高比容量、1.25 V 的工作电压以及在 200 mA g -1的电流密度下循环 1000 次后容量保持率 >98% 。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号