当前位置:
X-MOL 学术
›
J. Polym. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic effect of DMSO in metal-catalyzed radical polymerization mediated by disproportionation facilitates living and immortal radical polymerizations
Journal of Polymer Science ( IF 3.9 ) Pub Date : 2023-02-06 , DOI: 10.1002/pol.20220632 Devendra S. Maurya 1 , Jasper Adamson 1, 2 , Nabil Bensabeh 3 , Gerard Lligadas 3 , Virgil Percec 1
Journal of Polymer Science ( IF 3.9 ) Pub Date : 2023-02-06 , DOI: 10.1002/pol.20220632 Devendra S. Maurya 1 , Jasper Adamson 1, 2 , Nabil Bensabeh 3 , Gerard Lligadas 3 , Virgil Percec 1
Affiliation
|
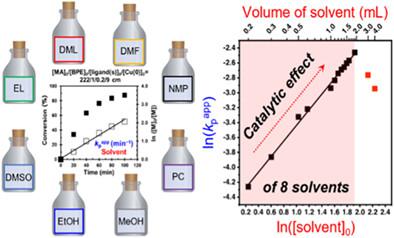
DMSO, an interesting solvent for copper-catalyzed living radical polymerization (LRP) mediated by disproportionation, does not exhibit the greatest disproportionation of Cu(I)X into Cu(0) and Cu(II)X2. Under suitable conditions, DMSO provides 100% conversion and absence of termination, facilitating the development of complex-architecture methodologies by living and immortal polymerizations. The mechanism yielding this level of precision is being investigated. Here we compare Cu(0)-wire-catalyzed LRP of methyl acrylate mediated by disproportionating ligands tris(2-dimethylaminoethyl)amine, Me6-TREN, tris(2-aminoethyl)amine, TREN, and Me6-TREN/TREN = 1/1 in presence of eight disproportionating solvents, some more efficient than DMSO in disproportionation. Unexpectedly, we observed that all solvents increased the rate of polymerization when monomer concentration decreased. This reversed trend from that of conventional LRPs demonstrates catalytic effect for disproportionating solvents. Above a certain concentration, the classic concentration-rate dependence was observed. The external order of reaction of the apparent rate constant of propagation, kpapp on solvent concentration demonstrated the highest order of reaction for the least disproportionating DMSO. Of all solvents investigated, DMSO has the highest ability to stabilize Cu(0) nanoparticles and therefore, yields the highest activity of Cu(0) nanoparticles rather than their greatest concentration. The implications of the catalytic effect of solvent in this and other reactions were discussed.
中文翻译:

DMSO 在歧化介导的金属催化自由基聚合中的催化作用促进了活性和不朽自由基聚合
DMSO 是一种有趣的溶剂,用于通过歧化作用介导的铜催化活性自由基聚合 (LRP),它不会将 Cu(I)X 最大程度地歧化为 Cu(0) 和 Cu(II)X 2。在合适的条件下,DMSO 可提供 100% 的转化率和终止作用,促进通过活性和不朽聚合开发复杂结构方法。产生这种精度水平的机制正在研究中。在这里,我们比较了由歧化配体三(2-二甲基氨基乙基)胺、Me 6 -TREN、三(2-氨基乙基)胺、TREN 和 Me 6介导的 Cu(0)-线催化的丙烯酸甲酯 LRP-TREN/TREN = 1/1,存在八种歧化溶剂,其中一些溶剂的歧化效率高于 DMSO。出乎意料的是,我们观察到当单体浓度降低时,所有溶剂都会增加聚合速率。这种与传统 LRP 相反的趋势证明了对歧化溶剂的催化作用。在一定浓度以上,观察到经典的浓度-速率依赖性。表观传播速率常数的外级反应,k p app对溶剂浓度的影响表明歧化最少的 DMSO 的反应顺序最高。在调查的所有溶剂中,DMSO 具有最高的稳定 Cu(0) 纳米粒子的能力,因此产生最高活性的 Cu(0) 纳米粒子,而不是它们的最大浓度。讨论了溶剂在该反应和其他反应中的催化作用的含义。
更新日期:2023-02-06
中文翻译:

DMSO 在歧化介导的金属催化自由基聚合中的催化作用促进了活性和不朽自由基聚合
DMSO 是一种有趣的溶剂,用于通过歧化作用介导的铜催化活性自由基聚合 (LRP),它不会将 Cu(I)X 最大程度地歧化为 Cu(0) 和 Cu(II)X 2。在合适的条件下,DMSO 可提供 100% 的转化率和终止作用,促进通过活性和不朽聚合开发复杂结构方法。产生这种精度水平的机制正在研究中。在这里,我们比较了由歧化配体三(2-二甲基氨基乙基)胺、Me 6 -TREN、三(2-氨基乙基)胺、TREN 和 Me 6介导的 Cu(0)-线催化的丙烯酸甲酯 LRP-TREN/TREN = 1/1,存在八种歧化溶剂,其中一些溶剂的歧化效率高于 DMSO。出乎意料的是,我们观察到当单体浓度降低时,所有溶剂都会增加聚合速率。这种与传统 LRP 相反的趋势证明了对歧化溶剂的催化作用。在一定浓度以上,观察到经典的浓度-速率依赖性。表观传播速率常数的外级反应,k p app对溶剂浓度的影响表明歧化最少的 DMSO 的反应顺序最高。在调查的所有溶剂中,DMSO 具有最高的稳定 Cu(0) 纳米粒子的能力,因此产生最高活性的 Cu(0) 纳米粒子,而不是它们的最大浓度。讨论了溶剂在该反应和其他反应中的催化作用的含义。

































 京公网安备 11010802027423号
京公网安备 11010802027423号