当前位置:
X-MOL 学术
›
ACS Eng. Au
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermodynamics, Kinetics, Morphology, and Raman studies for sH Hydrate of Methane and Cyclooctane
ACS Engineering Au ( IF 4.3 ) Pub Date : 2023-02-06 , DOI: 10.1021/acsengineeringau.2c00050 Namrata Gaikwad 1, 2 , Hyunho Kim 1 , Gaurav Bhattacharjee 1 , Jitendra S Sangwai 2 , Rajnish Kumar 2 , Praveen Linga 1
ACS Engineering Au ( IF 4.3 ) Pub Date : 2023-02-06 , DOI: 10.1021/acsengineeringau.2c00050 Namrata Gaikwad 1, 2 , Hyunho Kim 1 , Gaurav Bhattacharjee 1 , Jitendra S Sangwai 2 , Rajnish Kumar 2 , Praveen Linga 1
Affiliation
|
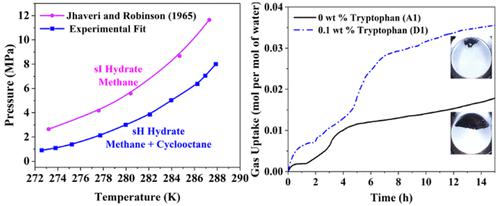
Natural gas is expected to be the major energy source in the near future, and storing it in the form of gas hydrate is a safe, clean, and economical approach. However, required thermodynamic conditions and slow kinetics are the key challenges that need to address for process viability. This study involves an experimental investigation of methane and cyclooctane sH hydrate formation for possible applications in gas storage using thermodynamics, kinetics, morphology, and Raman analysis. The hydrate formation is carried out at such thermodynamic conditions where only sH hydrate would form. The four-phase (Lw-LHC-H-V) sH hydrate equilibrium is studied for the methane and cyclooctane system via dissociation along the phase boundary method which is a robust method as it delivers a greater number of equilibrium data points in a single experimental run compared to other available methods. The sH hydrate formation helps in lowering the equilibrium conditions compared with sI hydrate formation. The slow sH hydrate formation kinetics can be improved by using low tryptophan concentrations. In this work, 0.1 wt % is the optimum tryptophan concentration as the gas uptake, and the hydrate formation rate is found to be the highest compared to 0.01, 0.05, and 1 wt % tryptophan concentrations. Here, we also visually investigate the sH hydrate formation and observed that the hydrate formation occurs below the interface for the system with no tryptophan; however, hydrate formation occurrence above the interface increases with an increase in the tryptophan concentration. The increase in the hydrate formation could be dedicated to the increased gas uptake due to the increasingly porous nature of hydrate formation. The Raman analysis confirmed the presence of methane and cyclooctane in sH hydrate cages. The higher intensity of the peaks using tryptophan additionally confirms the higher hydrate formation compared to the system with no tryptophan.
中文翻译:

甲烷和环辛烷的 sH 水合物的热力学、动力学、形态学和拉曼研究
天然气预计将在不久的将来成为主要能源,而以天然气水合物的形式储存天然气是一种安全、清洁且经济的方法。然而,所需的热力学条件和慢动力学是工艺可行性需要解决的关键挑战。本研究涉及利用热力学、动力学、形态和拉曼分析对甲烷和环辛烷 sH 水合物形成的可能应用进行气体储存的实验研究。水合物的形成是在仅形成sH水合物的热力学条件下进行的。四相(L w -L HC-HV) sH 水合物平衡是通过沿相界法解离来研究甲烷和环辛烷系统的,这是一种稳健的方法,因为与其他可用方法相比,它在单次实验运行中提供了更多数量的平衡数据点。与 sI 水合物形成相比,sH 水合物形成有助于降低平衡条件。通过使用低色氨酸浓度可以改善缓慢的 sH 水合物形成动力学。在这项工作中,0.1 wt% 是气体吸收的最佳色氨酸浓度,并且发现与 0.01、0.05 和 1 wt% 色氨酸浓度相比,水合物形成速率最高。在这里,我们还直观地研究了 sH 水合物的形成,并观察到水合物的形成发生在不含色氨酸的系统的界面下方;然而,界面上方水合物形成的发生随着色氨酸浓度的增加而增加。水合物形成的增加可能是由于水合物形成的多孔性质增加而导致气体吸收的增加。拉曼分析证实了 sH 水合物笼中存在甲烷和环辛烷。与没有色氨酸的系统相比,使用色氨酸的峰强度更高,另外证实了更高的水合物形成。
更新日期:2023-02-06
中文翻译:

甲烷和环辛烷的 sH 水合物的热力学、动力学、形态学和拉曼研究
天然气预计将在不久的将来成为主要能源,而以天然气水合物的形式储存天然气是一种安全、清洁且经济的方法。然而,所需的热力学条件和慢动力学是工艺可行性需要解决的关键挑战。本研究涉及利用热力学、动力学、形态和拉曼分析对甲烷和环辛烷 sH 水合物形成的可能应用进行气体储存的实验研究。水合物的形成是在仅形成sH水合物的热力学条件下进行的。四相(L w -L HC-HV) sH 水合物平衡是通过沿相界法解离来研究甲烷和环辛烷系统的,这是一种稳健的方法,因为与其他可用方法相比,它在单次实验运行中提供了更多数量的平衡数据点。与 sI 水合物形成相比,sH 水合物形成有助于降低平衡条件。通过使用低色氨酸浓度可以改善缓慢的 sH 水合物形成动力学。在这项工作中,0.1 wt% 是气体吸收的最佳色氨酸浓度,并且发现与 0.01、0.05 和 1 wt% 色氨酸浓度相比,水合物形成速率最高。在这里,我们还直观地研究了 sH 水合物的形成,并观察到水合物的形成发生在不含色氨酸的系统的界面下方;然而,界面上方水合物形成的发生随着色氨酸浓度的增加而增加。水合物形成的增加可能是由于水合物形成的多孔性质增加而导致气体吸收的增加。拉曼分析证实了 sH 水合物笼中存在甲烷和环辛烷。与没有色氨酸的系统相比,使用色氨酸的峰强度更高,另外证实了更高的水合物形成。































 京公网安备 11010802027423号
京公网安备 11010802027423号