Tetrahedron ( IF 2.1 ) Pub Date : 2023-02-02 , DOI: 10.1016/j.tet.2023.133296
Myunggi Jung 1 , Joanna E Muir 1 , Vincent N G Lindsay 1
|
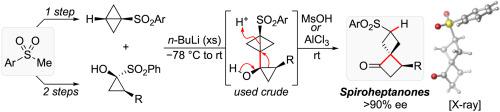
A novel approach for the formation of the highly strained spiro[3.3]heptan-1-one motif was developed through the reaction of 1-sulfonylcyclopropanols and lithiated 1-sulfonylbicyclo[1.1.0]butanes. Following initial nucleophilic addition to the cyclopropanone formed in situ, the resulting 1-bicyclobutylcyclopropanol intermediate is prone to a ‘strain-relocating’ semipinacol rearrangement in the presence of acid, directly affording the substituted spiro[3.3]heptan-1-one. The process is shown to be fully regio- and stereospecific when starting from a substituted cyclopropanone equivalent, leading to optically active 3-substituted spiro[3.3]heptan-1-ones. The reaction likely proceeds via initial protonation of the bicyclobutyl moiety followed by [1,2]-rearrangement of the resulting cyclopropylcarbinyl cation.
中文翻译:

通过菌株重定位半频哪醇重排方便合成螺[3.3]庚烷-1-酮
通过1-磺酰基环丙醇和锂化1-磺酰基双环[1.1.0]丁烷的反应,开发了一种形成高度紧张的螺[3.3]庚烷-1-酮基序的新方法。在原位形成的环丙酮上进行初始亲核加成后,所得的 1-二环丁基环丙醇中间体在酸存在下易于发生“应变重定位”半频哪醇重排,直接提供取代的螺[3.3]庚烷-1-酮。当从取代的环丙酮等价物开始时,该过程被证明是完全区域和立体特异性的,产生光学活性的3-取代的螺[3.3]庚-1-酮。该反应可能通过双环丁基部分的初始质子化和随后所得环丙基羰基阳离子的[1,2]-重排进行。



































 京公网安备 11010802027423号
京公网安备 11010802027423号