当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Decisive Role of Li2O2 Desorption for Oxygen Reduction Reaction in Li–O2 Batteries
ACS Energy Letters ( IF 19.3 ) Pub Date : 2023-02-03 , DOI: 10.1021/acsenergylett.2c02714 Chengyang Xu 1, 2 , Aimin Ge 2 , Koki Kannari 2 , Baoxu Peng 2 , Min Xue 1 , Bing Ding 1 , Ken-ichi Inoue 2 , Xiaogang Zhang 1 , Shen Ye 2, 3
ACS Energy Letters ( IF 19.3 ) Pub Date : 2023-02-03 , DOI: 10.1021/acsenergylett.2c02714 Chengyang Xu 1, 2 , Aimin Ge 2 , Koki Kannari 2 , Baoxu Peng 2 , Min Xue 1 , Bing Ding 1 , Ken-ichi Inoue 2 , Xiaogang Zhang 1 , Shen Ye 2, 3
Affiliation
|
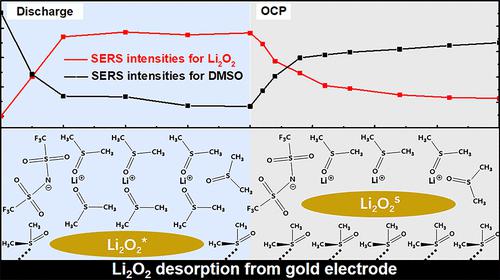
Fundamental issues relevant to the oxygen reduction reaction (ORR) mechanism and reaction interface are ambiguous in Li–O2 batteries. Herein, we utilized highly sensitive surface-enhanced Raman spectroscopy (SERS) to reveal the spontaneous desorption behavior of insoluble products of lithium peroxide (Li2O2) from the electrode surface. Furthermore, the electrochemical ORR mechanism is elucidated at the electrode/Li2O2 interface based on a dynamic equilibrium between the generation and desorption of Li2O2. The desorption of adsorbed Li2O2 species (Li2O2*) is crucial to releasing surface sites and maintaining the electrochemical ORR process at the electrode substrate surface instead of the Li2O2/electrolyte interface. The proceeding of Li2O2* desorption can guarantee the stability of Li2O2* concentration and the discharge plateau in the galvanostatic ORR process. The suppression of Li2O2* desorption is proved to induce the termination of Li2O2* generation, which is accompanied by the growth of adsorbed lithium superoxide (LiO2*), leading to rapid potential attenuation.
中文翻译:

Li2O2 脱附对 Li-O2 电池中氧还原反应的决定性作用
在 Li-O 2电池中,与氧还原反应 (ORR) 机制和反应界面相关的基本问题尚不明确。在此,我们利用高灵敏度表面增强拉曼光谱 (SERS) 来揭示过氧化锂 (Li 2 O 2 ) 不溶性产物从电极表面的自发解吸行为。此外,基于 Li 2 O 2的生成和解吸之间的动态平衡,在电极/Li 2 O 2 界面阐明了电化学ORR机制。吸附的 Li 2 O 2物种(Li 2 O 2*) 对于释放表面位点和维持电极基底表面而非 Li 2 O 2 /电解质界面处的电化学 ORR 过程至关重要。Li 2 O 2 * 脱附的进行可以保证恒流ORR过程中Li 2 O 2 * 浓度的稳定和放电平台。Li 2 O 2 * 解吸的抑制被证明可诱导Li 2 O 2 * 生成的终止,伴随着吸附的超氧化锂(LiO 2 *)的生长,导致电位快速衰减。
更新日期:2023-02-03
中文翻译:

Li2O2 脱附对 Li-O2 电池中氧还原反应的决定性作用
在 Li-O 2电池中,与氧还原反应 (ORR) 机制和反应界面相关的基本问题尚不明确。在此,我们利用高灵敏度表面增强拉曼光谱 (SERS) 来揭示过氧化锂 (Li 2 O 2 ) 不溶性产物从电极表面的自发解吸行为。此外,基于 Li 2 O 2的生成和解吸之间的动态平衡,在电极/Li 2 O 2 界面阐明了电化学ORR机制。吸附的 Li 2 O 2物种(Li 2 O 2*) 对于释放表面位点和维持电极基底表面而非 Li 2 O 2 /电解质界面处的电化学 ORR 过程至关重要。Li 2 O 2 * 脱附的进行可以保证恒流ORR过程中Li 2 O 2 * 浓度的稳定和放电平台。Li 2 O 2 * 解吸的抑制被证明可诱导Li 2 O 2 * 生成的终止,伴随着吸附的超氧化锂(LiO 2 *)的生长,导致电位快速衰减。

































 京公网安备 11010802027423号
京公网安备 11010802027423号