Journal of Membrane Science ( IF 8.4 ) Pub Date : 2023-02-01 , DOI: 10.1016/j.memsci.2023.121448 Su Liu , Xin Tong , Lei Huang , Chengchao Xiao , Kaihang Zhang , Yongsheng Chen , John Crittenden
|
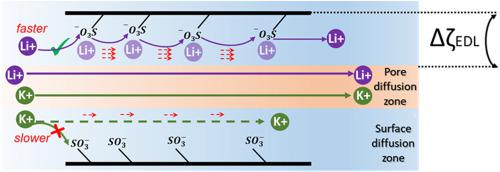
Lithium-ion extraction and recovery from unconventional aqueous sources by utilizing membranes as a separation barrier are potential technologies to address the challenge of increased lithium demand. Separating lithium from other competing metal ions is challenging due to their low selectivity when using conventional membranes as selective barriers. Graphene oxide (GO) nanofluidic membranes possess a higher monovalent ion permeation rate than multivalent ions, however, the selectivity of lithium over other monovalent ions is low. In this study, we created freestanding GO-based membranes with enhanced Li+/monovalent ions selectivity to concentrate and recover lithium from aqueous solutions containing competing monovalent ions (e.g., K+ and Na+). Specifically, sulfonate groups are introduced into the membrane matrices by intercalating the spacing agent, polystyrene sulfonate (PSS), into the adjacent GO nanosheets. Additionally, neutral and positively charged spacing agents are also used for comparison, and to further investigate the transport mechanisms. The Li+ permeation rate of GOM-S (PSS-incorporated GO membrane) is higher than the other GO-based membranes in a single salt solution. In a mixed salt solution, the Li+/K+ selectivity of GOM-S depends on concentration and we observed the highest value, 3.43, when using 0.5 M LiCl and 0.5 M KCl as the feed (high) concentrations and 0.001 M LiCl and 0.001 M KCl as permeate (low) concentrations. We observed that as permeate concentration decreased the selectivity increased and the pH values from 3 to 10 did not change the selectivity. DFT calculations show that the Li–SO3H pair has smaller binding energy, facilitating lithium-ion transport along the membrane nanochannel. Also, the solution concentration is crucial to the competitive mixed ionic transport, which results from the preferential surface transport induced by the electrical double layer (EDL) extension within the confined membrane nanochannels.
中文翻译:

使用电驱动独立式氧化石墨烯复合膜提取锂离子
利用膜作为分离屏障从非常规水源中提取和回收锂离子是应对锂需求增加挑战的潜在技术。将锂与其他竞争性金属离子分离具有挑战性,因为在使用传统膜作为选择性屏障时它们的选择性较低。氧化石墨烯 (GO) 纳米流体膜具有比多价离子更高的单价离子渗透率,但是,锂相对于其他单价离子的选择性较低。在这项研究中,我们创建了独立的基于 GO 的膜,具有增强的 Li + /单价离子选择性,以从含有竞争性单价离子(例如,K +和 Na +)的水溶液中浓缩和回收锂). 具体而言,通过将间隔剂聚苯乙烯磺酸盐 (PSS) 插入相邻的 GO 纳米片中,将磺酸盐基团引入膜基质中。此外,中性和带正电荷的间隔剂也用于比较,并进一步研究传输机制。GOM-S(结合 PSS 的 GO 膜)的 Li +渗透率在单一盐溶液中高于其他基于 GO 的膜。在混合盐溶液中,Li + /K +GOM-S 的选择性取决于浓度,当使用 0.5 M LiCl 和 0.5 M KCl 作为进料(高)浓度和 0.001 M LiCl 和 0.001 M KCl 作为渗透(低)浓度时,我们观察到最高值 3.43。我们观察到,随着渗透物浓度的降低,选择性增加,pH 值从 3 到 10 不会改变选择性。DFT 计算表明,Li–SO 3 H 对具有较小的结合能,有利于锂离子沿膜纳米通道的传输。此外,溶液浓度对于竞争性混合离子传输至关重要,这是由受限膜纳米通道内双电层 (EDL) 延伸引起的优先表面传输引起的。






























 京公网安备 11010802027423号
京公网安备 11010802027423号