European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2023-02-03 , DOI: 10.1016/j.ejmech.2023.115180 Mahmoud A Ragab 1 , Wagdy M Eldehna 2 , Alessio Nocentini 3 , Alessandro Bonardi 3 , Hazem E Okda 4 , Bahaa Elgendy 5 , Tarek S Ibrahim 6 , Mohammad M Abd-Alhaseeb 7 , Paola Gratteri 8 , Claudiu T Supuran 9 , Ahmed A Al-Karmalawy 10 , Mohamed Elagawany 1
|
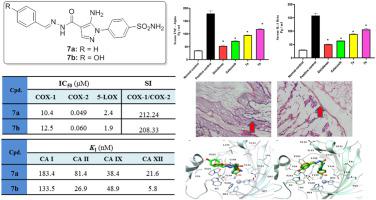
In the current medical era, the single target inhibition paradigm of drug discovery has given way to the multi-target design concept. As the most intricate pathological process, inflammation gives rise to a variety of diseases. There are several drawbacks to the single target anti-inflammatory drugs currently available. Herein, we present the design and synthesis of a novel series of 4-(5-amino-pyrazol-1-yl)benzenesulfonamide derivatives (7a-j) with COX-2, 5-LOX and carbonic anhydrase (CA) inhibitory activities as potential multi-target anti-inflammatory agents. The pharmacophoric 4-(pyrazol-1-yl)benzenesulfonamide moiety in Celecoxib was used as the core scaffold and different substituted phenyl and 2-thienyl tails were grafted via a hydrazone linker to enhance inhibitory activity against hCA IX and XII isoforms, yielding target pyrazoles 7a-j. All reported pyrazoles were evaluated for their inhibitory activity against COX-1, COX-2, and 5-LOX. Pyrazoles 7a, 7b, and 7j showed the best inhibitory activities against the COX-2 isozyme (IC50 = 49, 60 and 60 nM, respectively) and against 5-LOX (IC50 = 2.4, 1.9, and 2.5 μM, respectively) with excellent SI indices (COX-1/COX-2) of 212.24, 208.33, and 158.33, respectively. In addition, the inhibitory activities of pyrazoles 7a-j were evaluated against four different hCA isoforms I, II, IX, and XII. Both transmembrane hCA IX and XII isoforms were potently inhibited by pyrazoles 7a-j with KI values in the nanomolar range; 13.0–82.1 nM and 5.8–62.0 nM, respectively. Furthermore, pyrazoles 7a and 7b with the highest COX-2 activity and selectivity indices were evaluated in vivo for their analgesic, anti-inflammatory, and ulcerogenic activities. The serum level of the inflammatory mediators was then measured in order to confirm the anti-inflammatory activities of pyrazoles 7a and 7b.
中文翻译:

4-(5-氨基-吡唑-1-基)苯磺酰胺衍生物作为新型多靶点抗炎剂,具有对 COX-2、5-LOX 和碳酸酐酶的抑制活性:设计、合成和生物学评估
在当前的医学时代,药物发现的单靶点抑制范式已经让位于多靶点设计概念。炎症作为最复杂的病理过程,引发多种疾病。目前可用的单一靶点抗炎药物存在一些缺点。在此,我们展示了一系列新型 4-(5-氨基-吡唑-1-基)苯磺酰胺衍生物 ( 7a-j ) 的设计和合成,其具有 COX-2、5-LOX 和碳酸酐酶 (CA) 抑制活性:潜在的多靶点抗炎药。以塞来昔布中药效基团4-(吡唑-1-基)苯磺酰胺部分为核心支架,通过腙连接体接枝不同取代的苯基和2-噻吩基尾部,以增强对h CA IX和XII异构体的抑制活性,产生目标吡唑7a-j 。所有报告的吡唑类药物均针对 COX-1、COX-2 和 5-LOX 的抑制活性进行了评估。吡唑7a 、 7b和7j对 COX-2 同工酶(IC 50分别为 49、60 和 60 nM)和 5-LOX(IC 50分别为 2.4、1.9 和 2.5 μM)表现出最佳的抑制活性。其 SI 指数 (COX-1/COX-2) 分别为 212.24、208.33 和 158.33。此外,还评估了吡唑7a-j对四种不同h CA 异构体I 、 II 、 IX和XII的抑制活性。 跨膜h CA IX和XII亚型均被吡唑7a-j有效抑制, K I值在纳摩尔范围内;分别为 13.0–82.1 nM 和 5.8–62.0 nM。此外,还对具有最高COX-2活性和选择性指数的吡唑7a和7b的镇痛、抗炎和溃疡活性进行了体内评估。然后测量炎症介质的血清水平以确认吡唑7a和7b的抗炎活性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号