当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photoredox/HAT-Catalyzed Dearomative Nucleophilic Addition of the CO2 Radical Anion to (Hetero)Aromatics
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-02-03 , DOI: 10.1021/acscatal.2c06192 Saeesh R. Mangaonkar 1, 2 , Hiroki Hayashi 1, 2 , Hideaki Takano 1, 2 , Wataru Kanna 3 , Satoshi Maeda 1, 2, 3, 4 , Tsuyoshi Mita 1, 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2023-02-03 , DOI: 10.1021/acscatal.2c06192 Saeesh R. Mangaonkar 1, 2 , Hiroki Hayashi 1, 2 , Hideaki Takano 1, 2 , Wataru Kanna 3 , Satoshi Maeda 1, 2, 3, 4 , Tsuyoshi Mita 1, 2
Affiliation
|
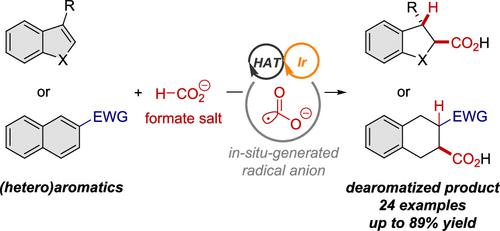
The radical anion of CO2 (CO2•–) is a strongly nucleophilic radical species with rapidly emerging applications in contemporary organic chemistry. This radical species exhibits high reactivity in single-electron reduction reactions due to the concomitant release of stable CO2, or Giese-type reactions, especially for electron-deficient alkenes and styrene derivatives. In contrast to previous reports, we herein disclose the development of a robust method for the introduction of CO2•–, which can be generated from cesium formate under photoredox/hydrogen atom transfer (HAT) catalysis, into stable heteroaromatics such as benzofuran, benzothiophene, and indole derivatives to afford synthetically useful α-oxy, α-thio, and α-amino acid derivatives in moderate to high yield. In addition, when using electron-deficient naphthalene derivatives, both single-electron reduction and Giese-type nucleophilic addition occur simultaneously to produce carboxylated tetrahydronaphthalene derivatives in good yield. Moreover, one of the tetrahydronaphthalenes that bear a cyano group was transformed into the corresponding γ-butyrolactam via reduction of the cyano functionality through hydrogenation followed by cyclization. To the best of our knowledge, these dearomative carboxylation reactions with metal formates under photoredox/HAT conditions are unprecedented, thus providing a synthetic option for the introduction of a C1 source into stable (hetero)aromatics.
中文翻译:

光氧化还原/HAT 催化的 CO2 自由基阴离子与(杂)芳烃的亲核加成反应
CO 2的自由基阴离子(CO 2 •– ) 是一种强亲核自由基,在现代有机化学中应用迅速。由于伴随释放稳定的 CO 2或 Giese 型反应,这种自由基物种在单电子还原反应中表现出高反应性,尤其是对于缺电子烯烃和苯乙烯衍生物。与之前的报告相比,我们在此披露了引入 CO 2的稳健方法的开发•–,可在光氧化还原/氢原子转移 (HAT) 催化下由甲酸铯生成,转化为稳定的杂芳烃,如苯并呋喃、苯并噻吩和吲哚衍生物,以提供合成有用的 α-氧、α-硫代和 α-氨基酸衍生物中高产。此外,当使用缺电子萘衍生物时,单电子还原和Giese型亲核加成同时发生,可以高产率地制备羧化四氢萘衍生物。此外,一种带有氰基的四氢萘通过氢化和环化还原氰基官能团,转化为相应的γ-丁内酰胺。据我们所知,
更新日期:2023-02-03
中文翻译:

光氧化还原/HAT 催化的 CO2 自由基阴离子与(杂)芳烃的亲核加成反应
CO 2的自由基阴离子(CO 2 •– ) 是一种强亲核自由基,在现代有机化学中应用迅速。由于伴随释放稳定的 CO 2或 Giese 型反应,这种自由基物种在单电子还原反应中表现出高反应性,尤其是对于缺电子烯烃和苯乙烯衍生物。与之前的报告相比,我们在此披露了引入 CO 2的稳健方法的开发•–,可在光氧化还原/氢原子转移 (HAT) 催化下由甲酸铯生成,转化为稳定的杂芳烃,如苯并呋喃、苯并噻吩和吲哚衍生物,以提供合成有用的 α-氧、α-硫代和 α-氨基酸衍生物中高产。此外,当使用缺电子萘衍生物时,单电子还原和Giese型亲核加成同时发生,可以高产率地制备羧化四氢萘衍生物。此外,一种带有氰基的四氢萘通过氢化和环化还原氰基官能团,转化为相应的γ-丁内酰胺。据我们所知,













































 京公网安备 11010802027423号
京公网安备 11010802027423号