当前位置:
X-MOL 学术
›
Energy Environ. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Striking Stabilization Effect of Spinel Cobalt Oxide Oxygen Evolution Electrocatalysts in Neutral pH by Dual-Sites Iron Incorporation
Energy & Environmental Materials ( IF 13.0 ) Pub Date : 2023-01-30 , DOI: 10.1002/eem2.12594 Shuairu Zhu 1, 2 , Xue Wang 1 , Jiabo Le 1 , Na An 1 , Jianming Li 1, 3 , Deyu Liu 1, 4 , Yongbo Kuang 1, 4
Energy & Environmental Materials ( IF 13.0 ) Pub Date : 2023-01-30 , DOI: 10.1002/eem2.12594 Shuairu Zhu 1, 2 , Xue Wang 1 , Jiabo Le 1 , Na An 1 , Jianming Li 1, 3 , Deyu Liu 1, 4 , Yongbo Kuang 1, 4
Affiliation
|
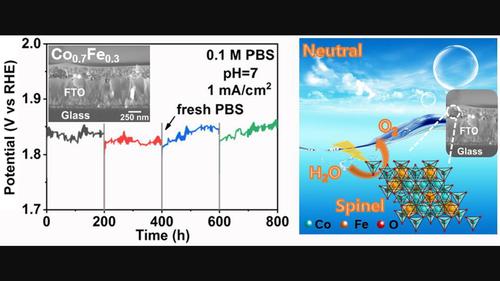
Developing stable and efficient nonprecious-metal-based oxygen evolution catalysts in the neutral electrolyte is a challenging but essential goal for various electrochemical systems. Particularly, cobalt-based spinels have drawn a considerable amount of attention but most of them operate in alkali solutions. However, the frequently studied Co–Fe spinel system never exhibits appreciable stability in nonbasic conditions, not to mention attract further investigation on its key structural motif and transition states for activity loss. Herein, we report exceptional stable Co–Fe spinel oxygen evolution catalysts (~30% Fe is optimal) in a neutral electrolyte, owing to its unique metal ion arrangements in the crystal lattice. The introduced iron content enters both the octahedral and tetrahedral sites of the spinel as Fe2+ and Fe3+ (with Co ions having mixed distribution as well). Combining density functional theory calculations, we find that the introduction of Fe to Co3O4 lowers the covalency of metal-oxygen bonds and can help suppress the oxidation of Co2+/3+ and O2−. It implies that the Co–Fe spinel will have minor surface reconstruction and less lattice oxygen loss during the oxygen evolution reaction process in comparison with Co3O4 and hence show much better stability. These findings suggest that there is still much chance for the spinel structures, especially using reasonable sublattices engineering via multimetal doping to develop advanced oxygen evolution catalysts.
中文翻译:

双位点铁掺入尖晶石钴氧化物析氧电催化剂在中性 pH 条件下的惊人稳定效果
在中性电解质中开发稳定、高效的非贵金属基析氧催化剂对于各种电化学系统来说是一个具有挑战性但又至关重要的目标。特别是钴基尖晶石引起了相当多的关注,但它们大多数在碱性溶液中使用。然而,经常研究的Co-Fe尖晶石体系在非碱性条件下从未表现出明显的稳定性,更不用说吸引对其关键结构基序和活性损失的过渡态的进一步研究。在此,我们报告了在中性电解质中异常稳定的 Co-Fe 尖晶石析氧催化剂(~30% Fe 是最佳),这归因于其晶格中独特的金属离子排列。引入的铁含量以Fe 2+和Fe 3+的形式进入尖晶石的八面体和四面体位点(Co离子也具有混合分布)。结合密度泛函理论计算,我们发现Co 3 O 4中Fe的引入降低了金属-氧键的共价键,有助于抑制Co 2+/3+和O 2−的氧化。这意味着与Co 3 O 4相比,Co-Fe尖晶石在析氧反应过程中具有较小的表面重构和较少的晶格氧损失,因此表现出更好的稳定性。这些发现表明尖晶石结构仍有很大的机会,特别是通过多金属掺杂使用合理的亚晶格工程来开发先进的析氧催化剂。
更新日期:2023-01-30
中文翻译:

双位点铁掺入尖晶石钴氧化物析氧电催化剂在中性 pH 条件下的惊人稳定效果
在中性电解质中开发稳定、高效的非贵金属基析氧催化剂对于各种电化学系统来说是一个具有挑战性但又至关重要的目标。特别是钴基尖晶石引起了相当多的关注,但它们大多数在碱性溶液中使用。然而,经常研究的Co-Fe尖晶石体系在非碱性条件下从未表现出明显的稳定性,更不用说吸引对其关键结构基序和活性损失的过渡态的进一步研究。在此,我们报告了在中性电解质中异常稳定的 Co-Fe 尖晶石析氧催化剂(~30% Fe 是最佳),这归因于其晶格中独特的金属离子排列。引入的铁含量以Fe 2+和Fe 3+的形式进入尖晶石的八面体和四面体位点(Co离子也具有混合分布)。结合密度泛函理论计算,我们发现Co 3 O 4中Fe的引入降低了金属-氧键的共价键,有助于抑制Co 2+/3+和O 2−的氧化。这意味着与Co 3 O 4相比,Co-Fe尖晶石在析氧反应过程中具有较小的表面重构和较少的晶格氧损失,因此表现出更好的稳定性。这些发现表明尖晶石结构仍有很大的机会,特别是通过多金属掺杂使用合理的亚晶格工程来开发先进的析氧催化剂。






























 京公网安备 11010802027423号
京公网安备 11010802027423号