当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Derivatized Benzothiazoles as Two-Photon-Absorbing Organic Photosensitizers Active under Near Infrared Light Irradiation
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-02-02 , DOI: 10.1021/jacs.2c12244
Bidyut Kumar Kundu 1 , Guanqun Han 1 , Yujie Sun 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2023-02-02 , DOI: 10.1021/jacs.2c12244
Bidyut Kumar Kundu 1 , Guanqun Han 1 , Yujie Sun 1
Affiliation
|
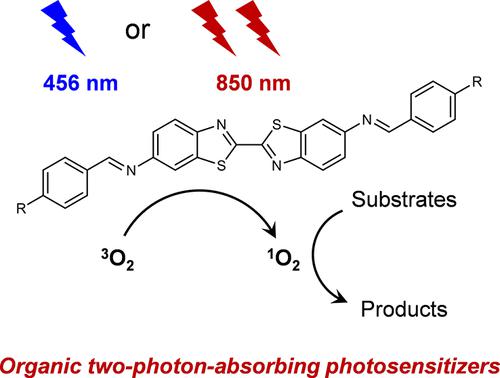
Homogeneous organic photocatalysis typically requires molecular photosensitizers absorbing in the ultraviolet–visible (UV/vis) region, because UV/vis photons possess the sufficient energy to excite those one-photon-absorbing photosensitizers to the desired excited states. However, UV/vis light irradiation has many potential limitations, especially for large-scale applications, such as low penetration through reaction media, competing absorption by substrates and co-catalysts, and incompatibility with substrates bearing light-sensitive functionalities. In fact, these drawbacks can be effectively avoided if near infrared (NIR) photons can be utilized to drive the target reactions. Herein, we report two benzothiazole-derived compounds as novel two-photon-absorbing (TPA) organic photosensitizers, which can function under NIR light irradiation using inexpensive LED as the light source. We demonstrate that by judicially modulating the donor−π–acceptor−π–donor-conjugated structure containing a bibenzothiazole core and imine bridges, excellent two-photon absorption capability in the NIR region can be achieved, approaching 2000 GM at 850 nm. Together with large quantum yields (∼0.5), these benzothiazole-derived TPA organic photosensitizers exhibit excellent performance in driving various O2-involved organic reactions upon irradiation at 850 nm, showing great penetration depth, superior to that upon blue light irradiation. A suite of photophysical and computational studies were performed to shed light on the underlying electronic states responsible for the observed TPA capability. Overall, this work highlights the promise of developing Ru/Ir-free organic photosensitizers operative in the NIR region by taking advantage of the two-photon absorption mechanism.
中文翻译:

衍生化苯并噻唑作为双光子吸收有机光敏剂在近红外光照射下具有活性
均相有机光催化通常需要在紫外-可见 (UV/vis) 区域吸收的分子光敏剂,因为 UV/vis 光子拥有足够的能量将那些单光子吸收光敏剂激发到所需的激发态。然而,紫外/可见光照射有许多潜在的局限性,特别是对于大规模应用,例如通过反应介质的低渗透性、底物和助催化剂的竞争吸收以及与具有光敏功能的底物的不相容性。事实上,如果可以利用近红外 (NIR) 光子来驱动目标反应,则可以有效避免这些缺点。在此,我们报道了两种苯并噻唑衍生化合物作为新型双光子吸收 (TPA) 有机光敏剂,它可以使用廉价的 LED 作为光源在 NIR 光照射下工作。我们证明,通过合理调制包含联苯并噻唑核和亚胺桥的供体-π-受体-π-供体共轭结构,可以在 NIR 区域实现出色的双光子吸收能力,在 850 nm 处接近 2000 GM。连同大量子产率 (~0.5),这些苯并噻唑衍生的 TPA 有机光敏剂在驱动各种 O2 方面表现出优异的性能2 -在850 nm照射下参与有机反应,表现出较大的穿透深度,优于蓝光照射。进行了一系列光物理和计算研究,以阐明导致观察到的 TPA 能力的潜在电子状态。总的来说,这项工作突出了利用双光子吸收机制开发在 NIR 区域起作用的无 Ru/Ir 有机光敏剂的前景。
更新日期:2023-02-02
中文翻译:

衍生化苯并噻唑作为双光子吸收有机光敏剂在近红外光照射下具有活性
均相有机光催化通常需要在紫外-可见 (UV/vis) 区域吸收的分子光敏剂,因为 UV/vis 光子拥有足够的能量将那些单光子吸收光敏剂激发到所需的激发态。然而,紫外/可见光照射有许多潜在的局限性,特别是对于大规模应用,例如通过反应介质的低渗透性、底物和助催化剂的竞争吸收以及与具有光敏功能的底物的不相容性。事实上,如果可以利用近红外 (NIR) 光子来驱动目标反应,则可以有效避免这些缺点。在此,我们报道了两种苯并噻唑衍生化合物作为新型双光子吸收 (TPA) 有机光敏剂,它可以使用廉价的 LED 作为光源在 NIR 光照射下工作。我们证明,通过合理调制包含联苯并噻唑核和亚胺桥的供体-π-受体-π-供体共轭结构,可以在 NIR 区域实现出色的双光子吸收能力,在 850 nm 处接近 2000 GM。连同大量子产率 (~0.5),这些苯并噻唑衍生的 TPA 有机光敏剂在驱动各种 O2 方面表现出优异的性能2 -在850 nm照射下参与有机反应,表现出较大的穿透深度,优于蓝光照射。进行了一系列光物理和计算研究,以阐明导致观察到的 TPA 能力的潜在电子状态。总的来说,这项工作突出了利用双光子吸收机制开发在 NIR 区域起作用的无 Ru/Ir 有机光敏剂的前景。

































 京公网安备 11010802027423号
京公网安备 11010802027423号